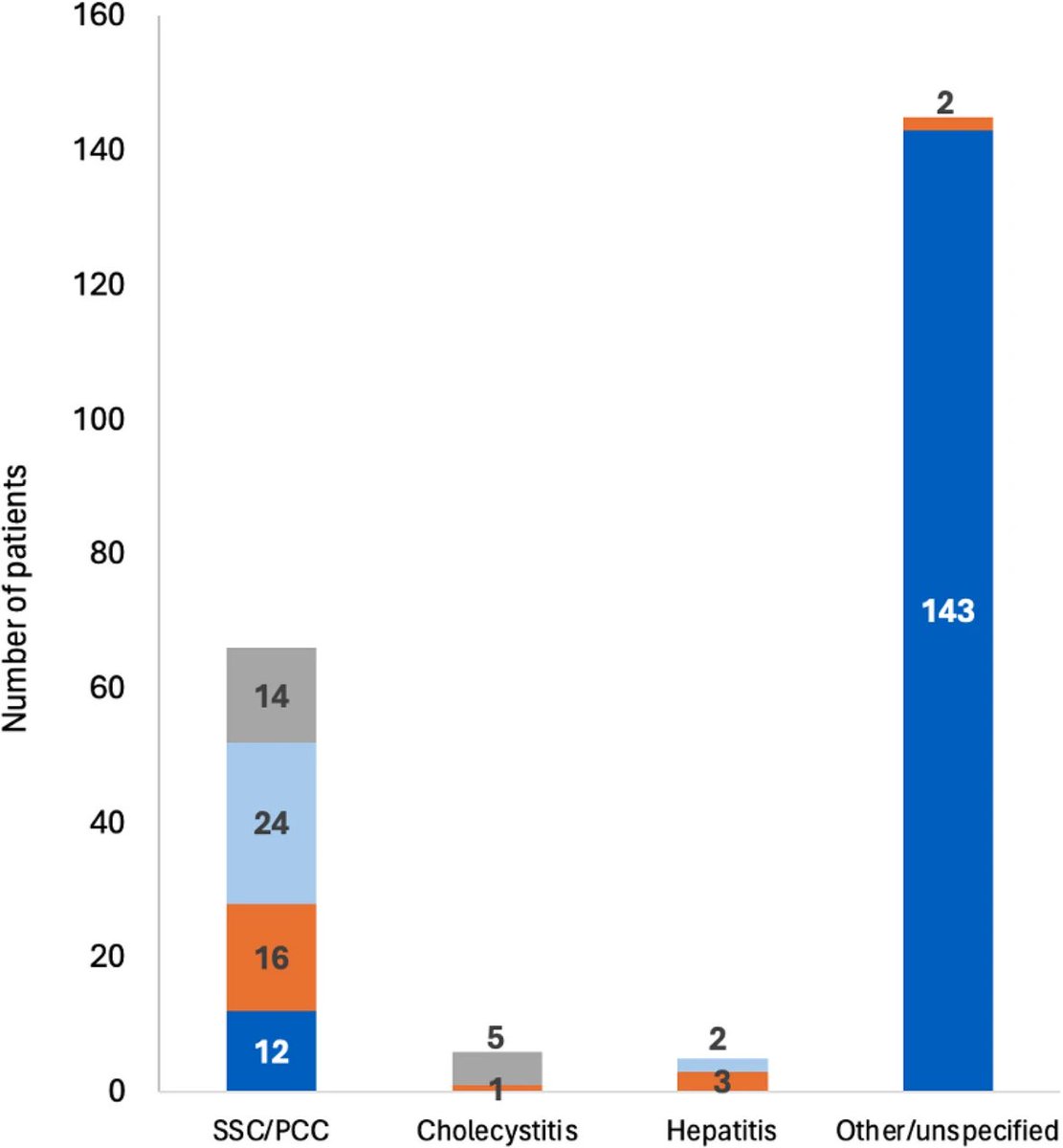
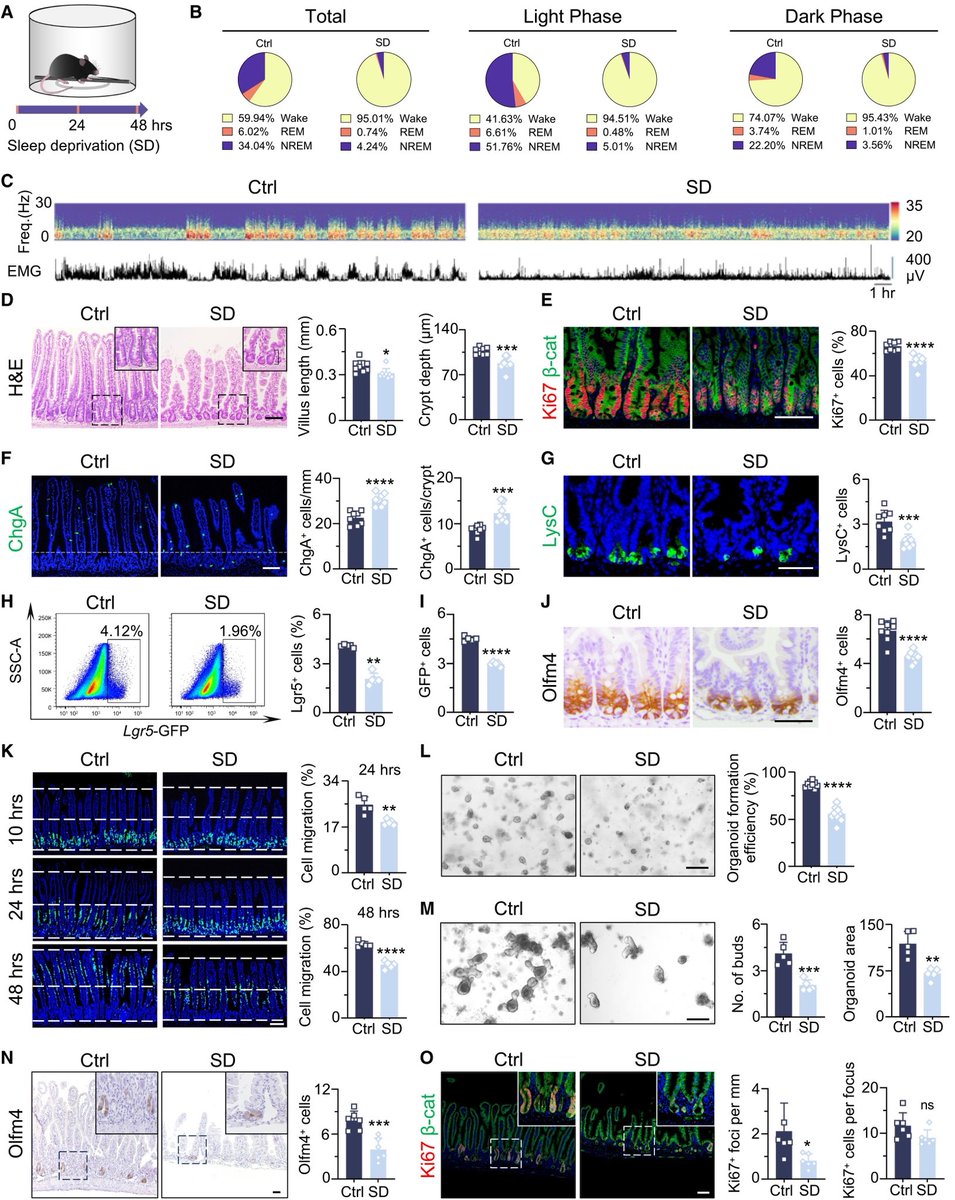
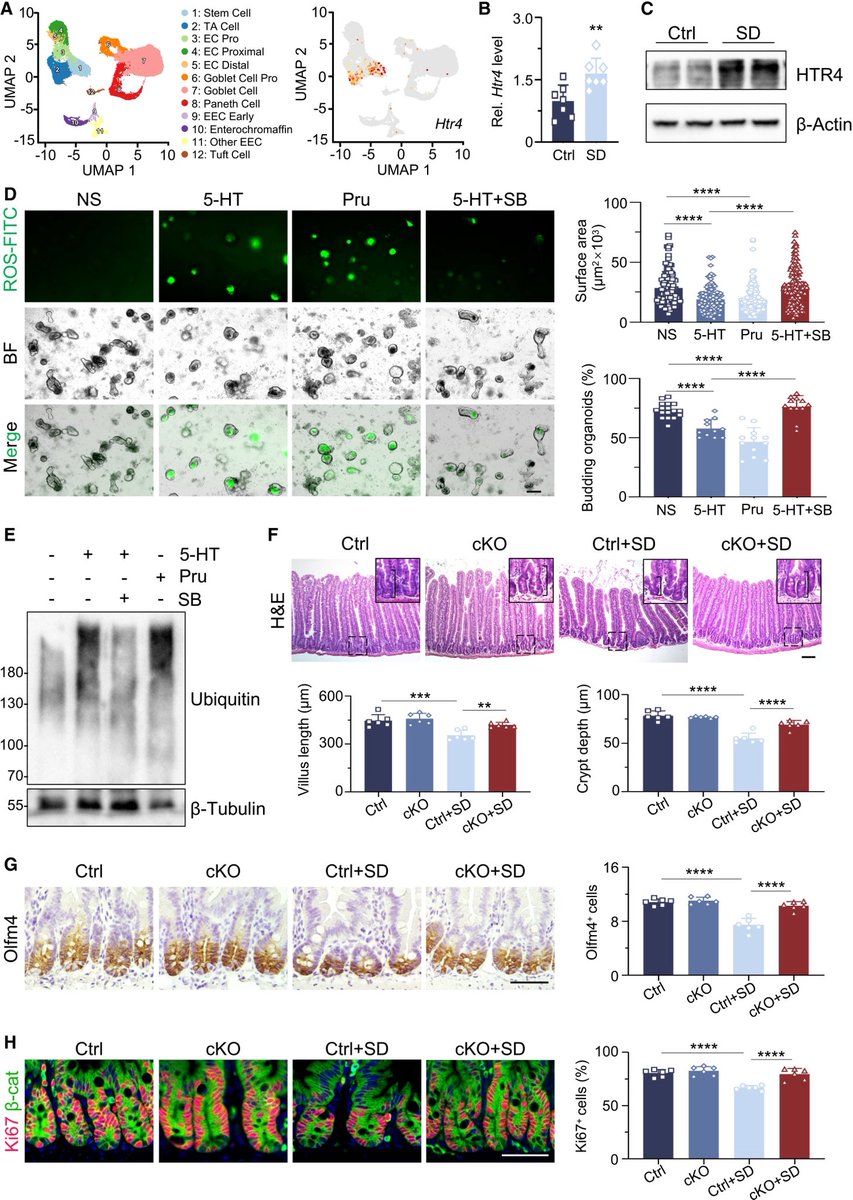
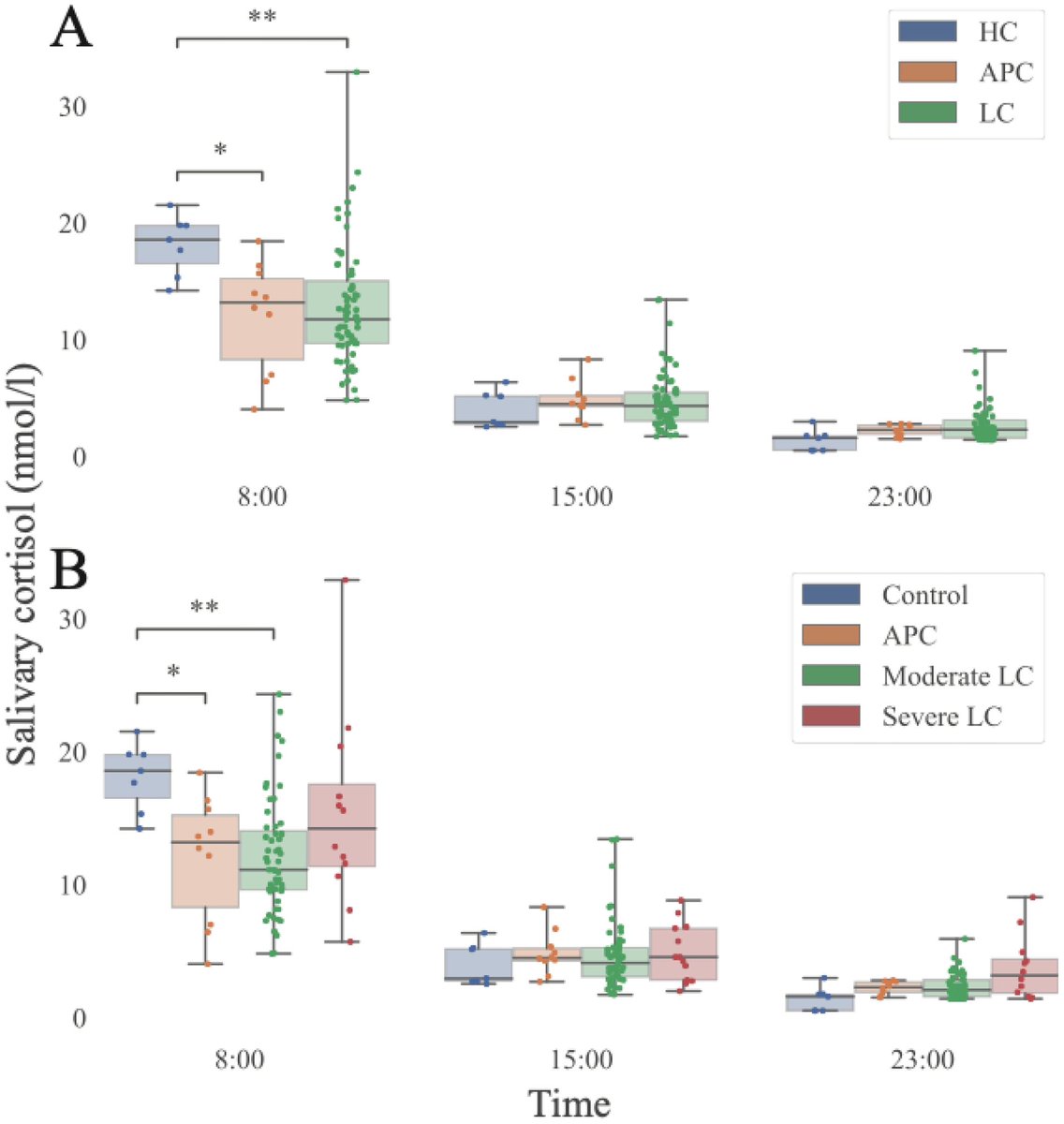
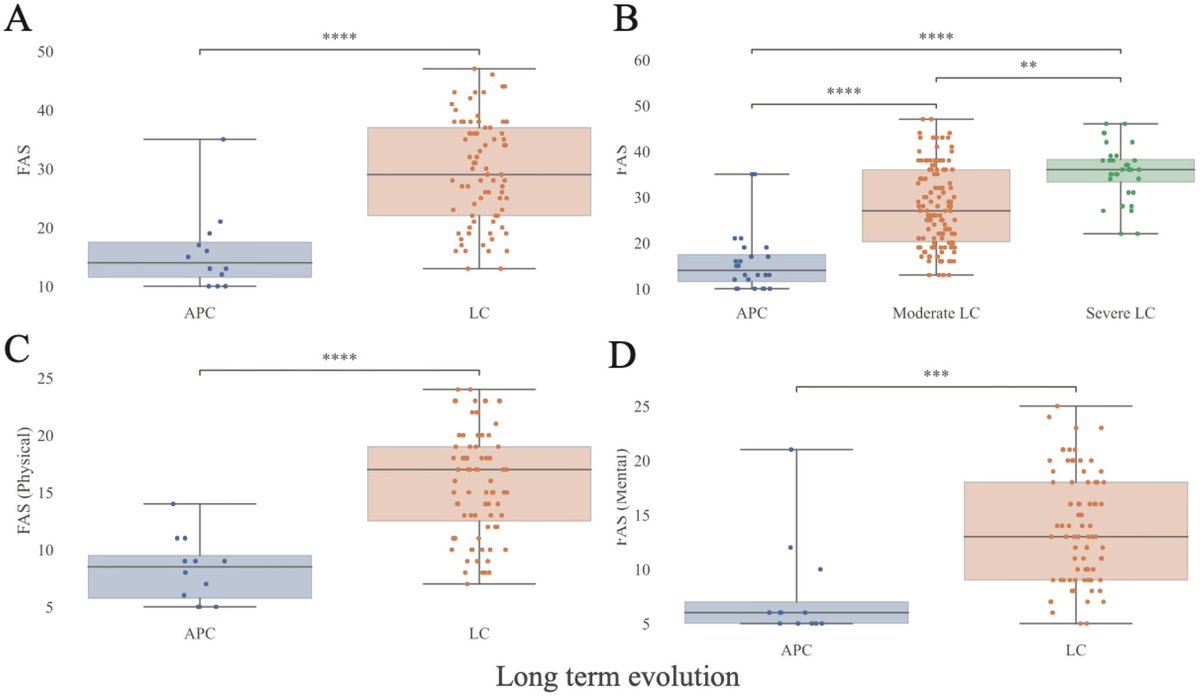
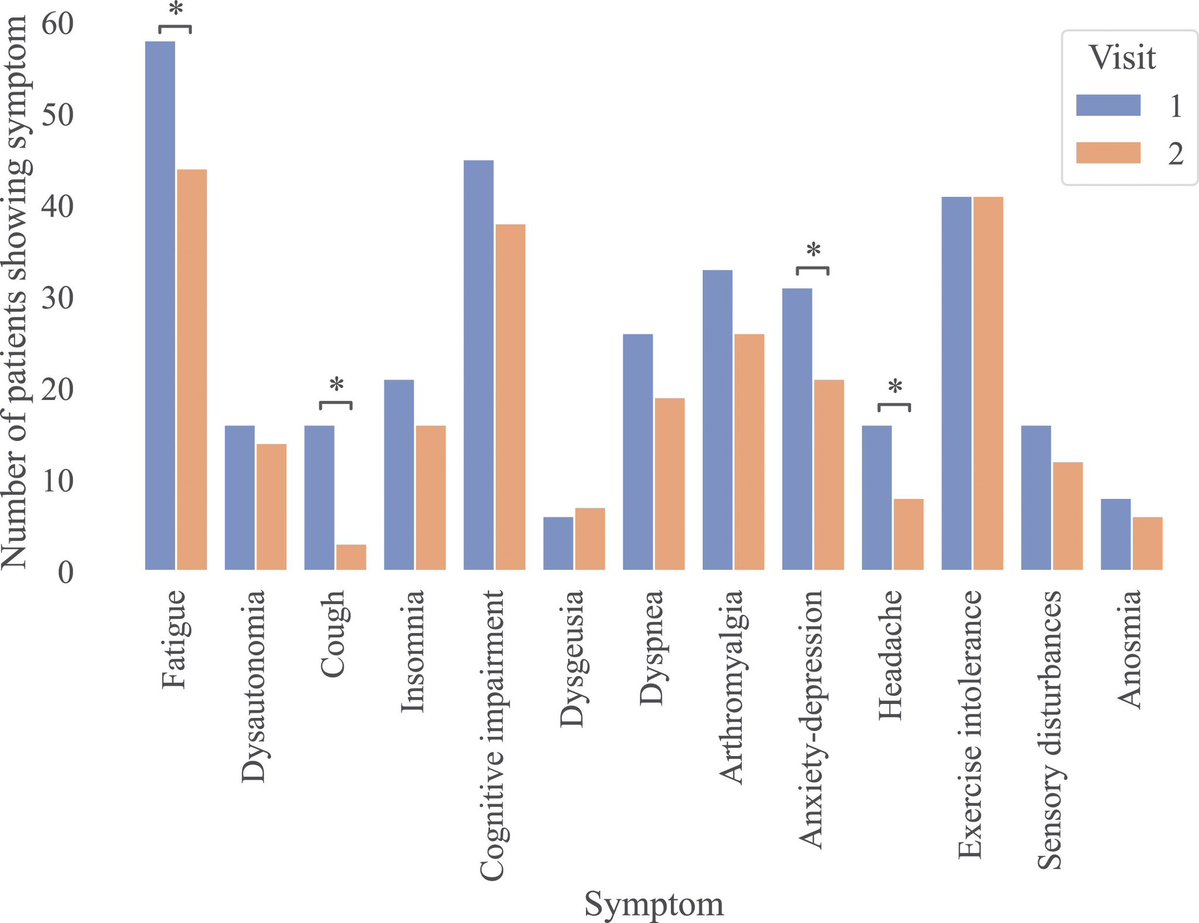
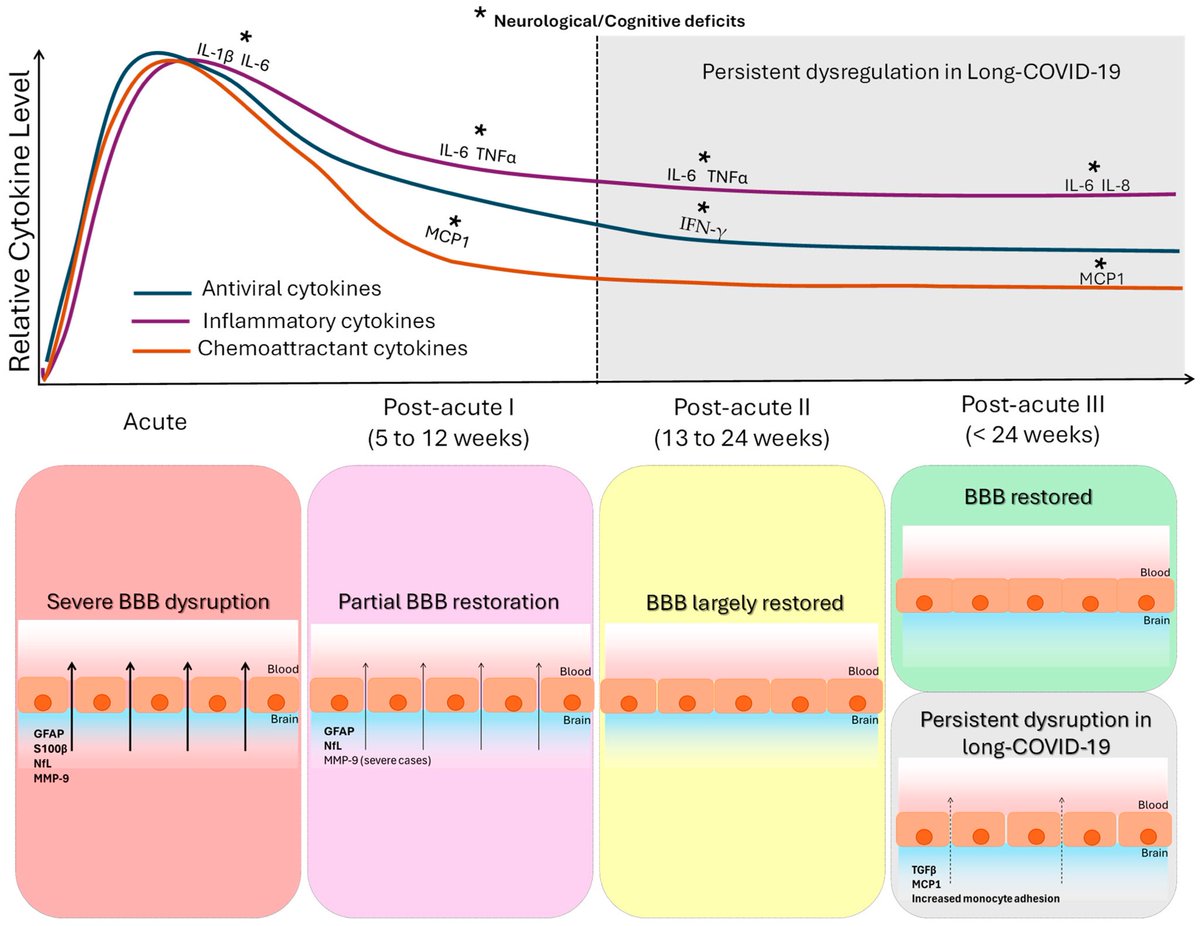
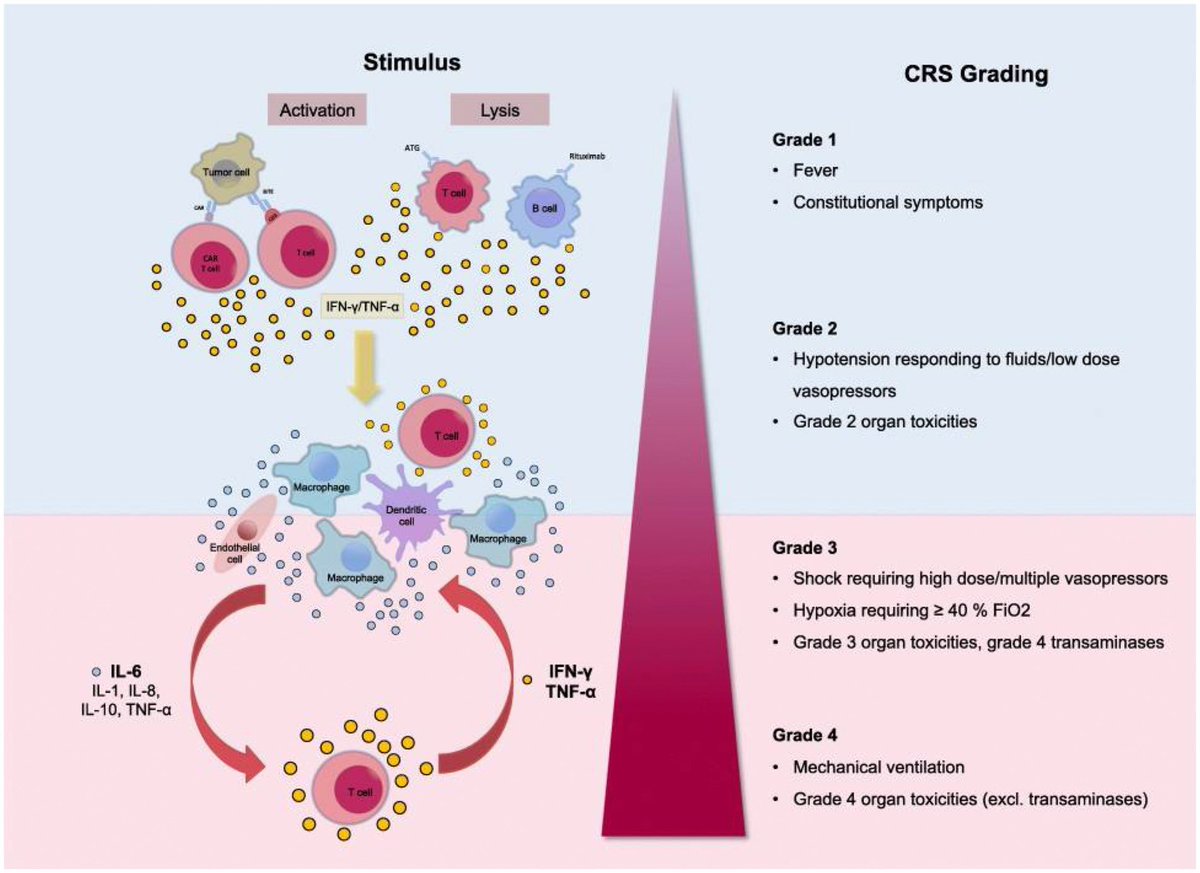
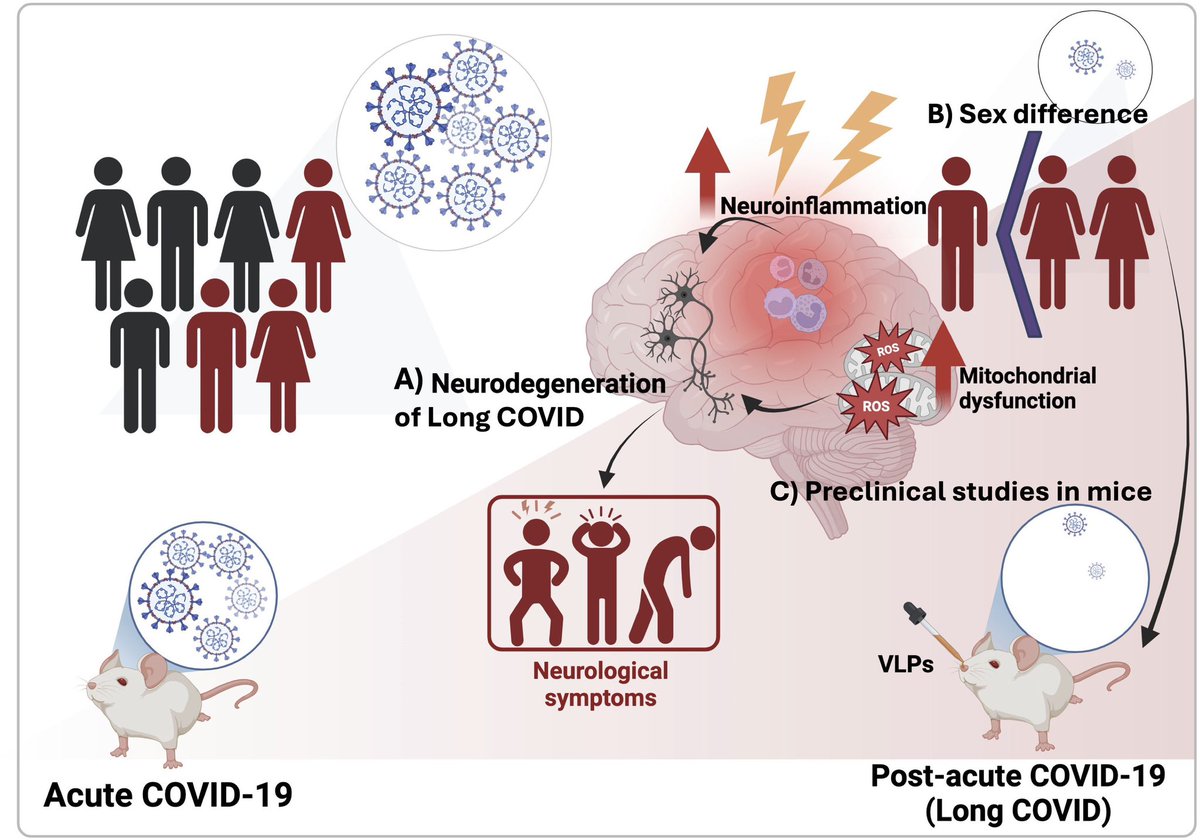
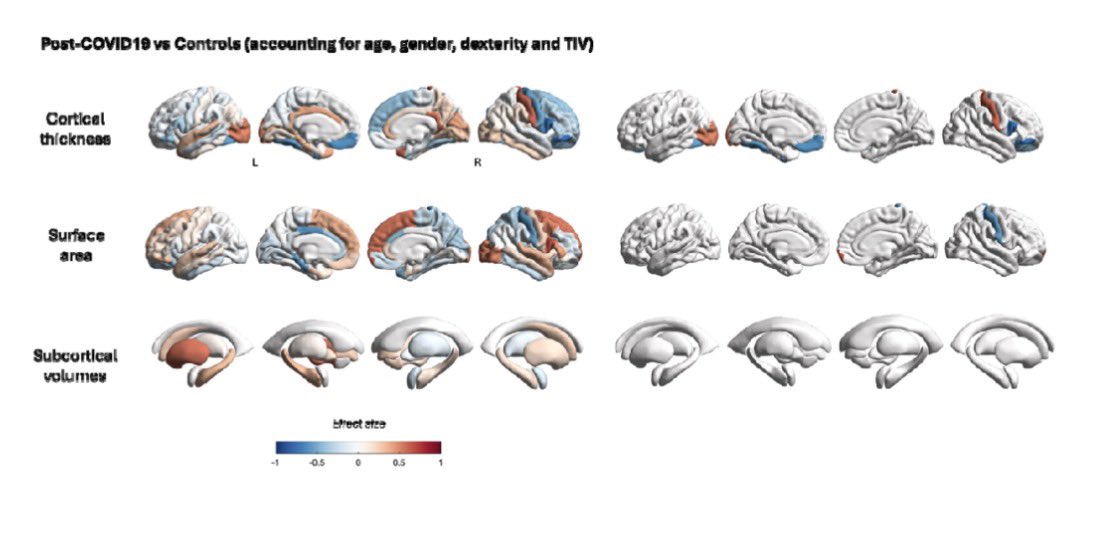
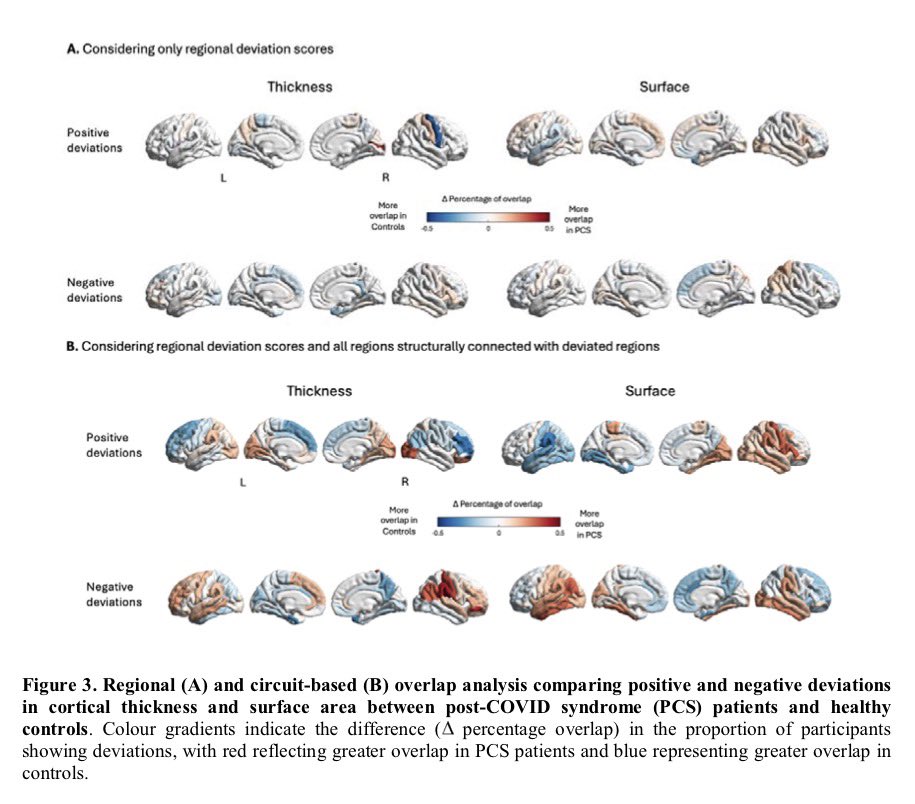
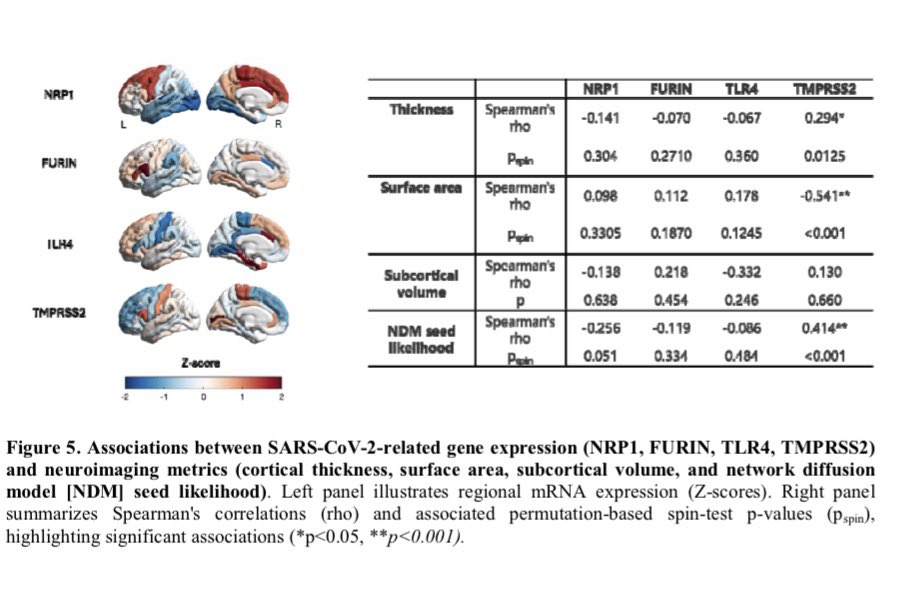
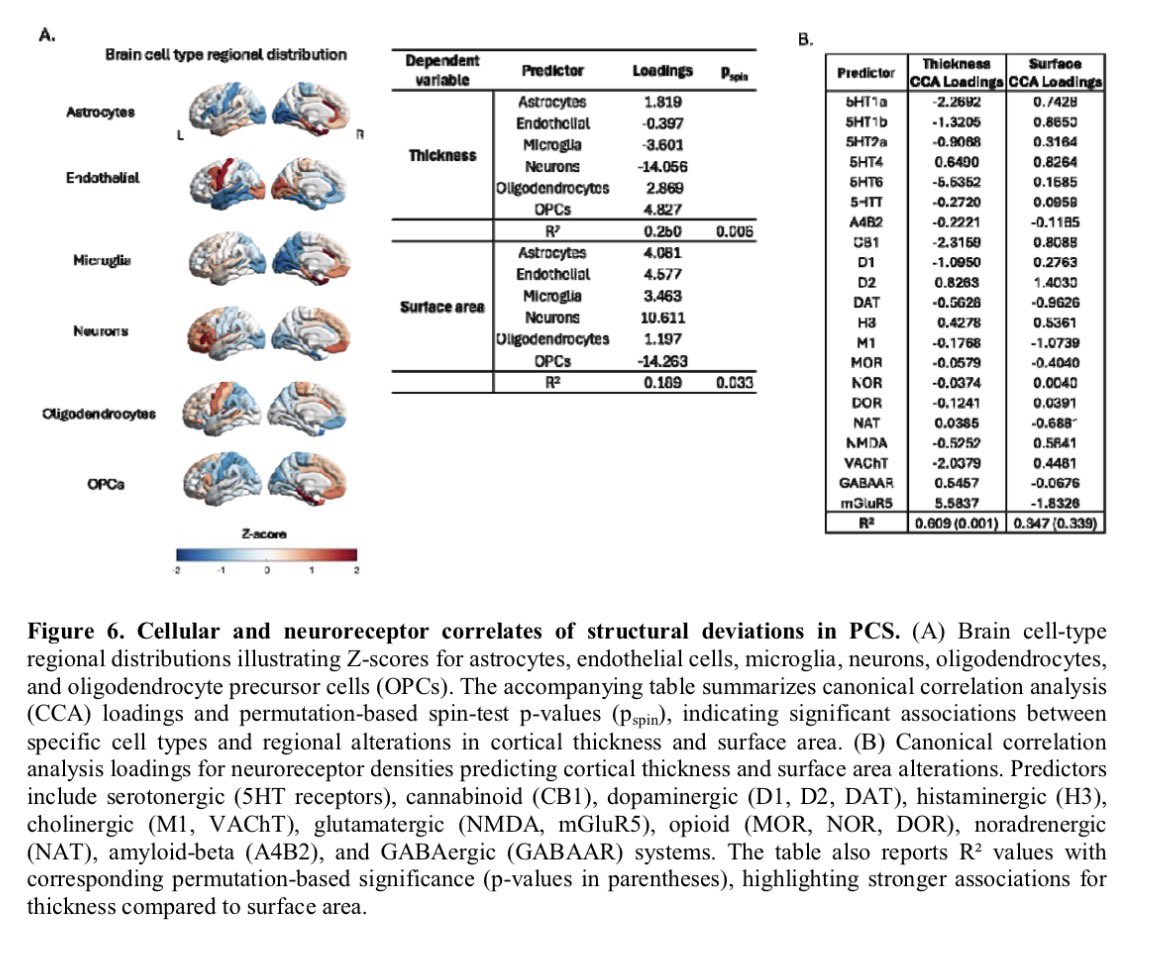
According to a new study, SARS-CoV-2 virus hijacks the machinery of testicular cells that produce the hormone testosterone in order to replicate.
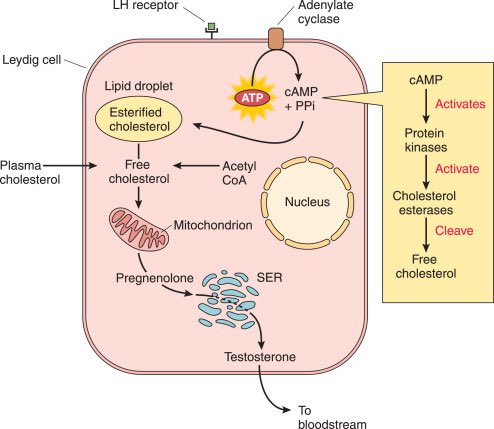
It also appropriates the metabolic pathways of these cells and cholesterol, a precursor of testosterone, thereby altering lipid metabolism for its formation. 1/
It also appropriates the metabolic pathways of these cells and cholesterol, a precursor of testosterone, thereby altering lipid metabolism for its formation. 1/

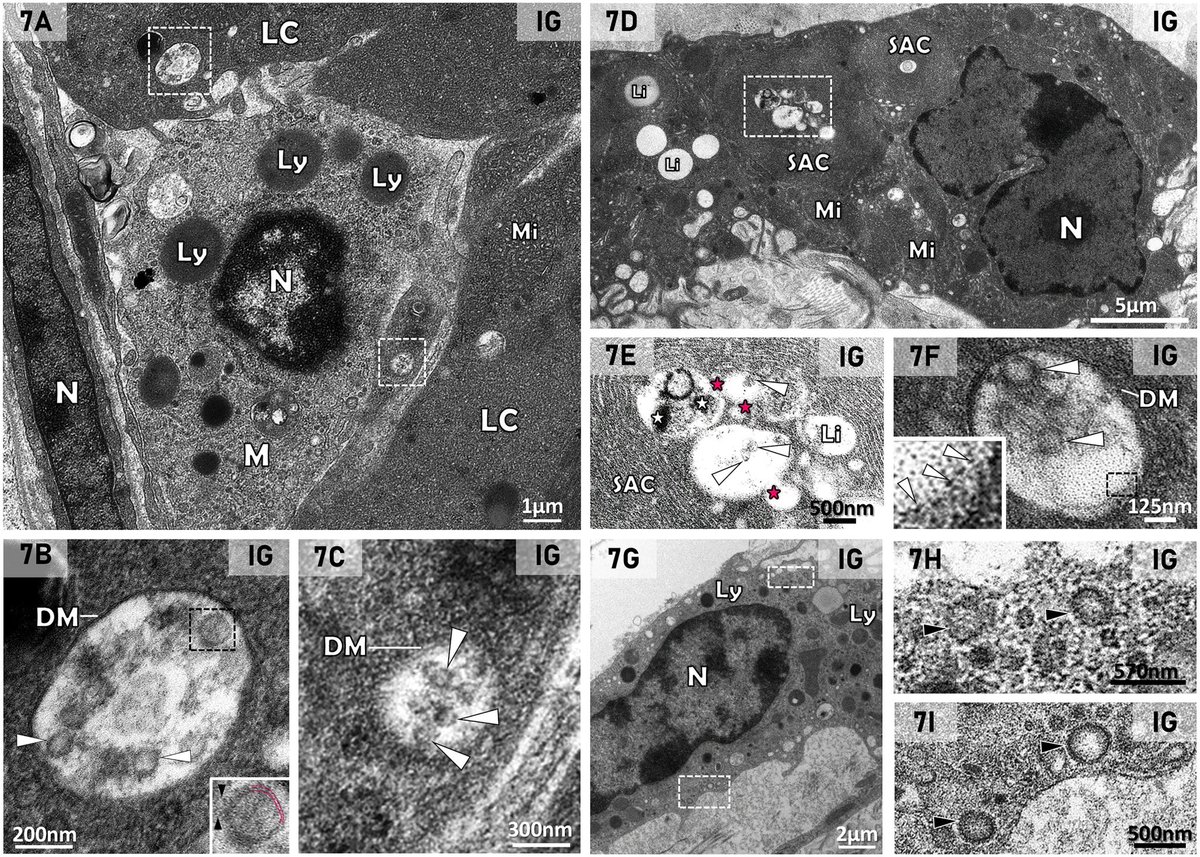
The study revealed the presence of SARS-CoV-2 particles in lipid inclusions and organelles responsible for testosterone production in Leydig cells for the first time.
In addition, the researchers described the mechanism by which the virus interferes with the functioning of these testicular cells.
The discovery helps explain why male patients with severe COVID-19 have lower levels of testosterone, and possibly cholesterol. 2/
In addition, the researchers described the mechanism by which the virus interferes with the functioning of these testicular cells.
The discovery helps explain why male patients with severe COVID-19 have lower levels of testosterone, and possibly cholesterol. 2/

After infecting the Leydig cells in the testicles, the virus uses lipid metabolism pathways and the cell structure to replicate, which impairs testosterone production.
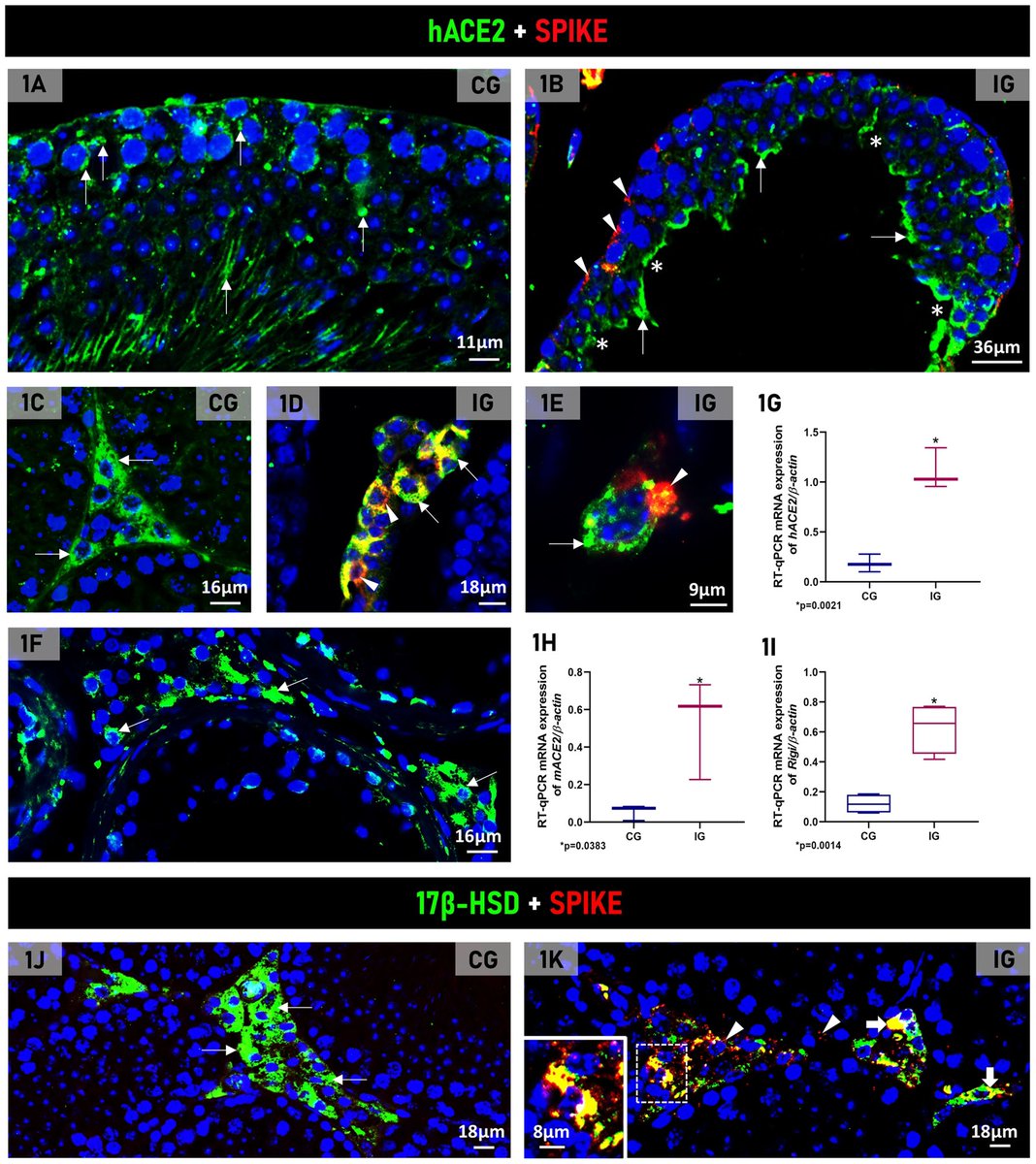
This happens because these cells, responsible for producing testosterone, express high concentrations of the ACE2 receptor, facilitating the entry of the virus, 3/
This happens because these cells, responsible for producing testosterone, express high concentrations of the ACE2 receptor, facilitating the entry of the virus, 3/

In addition, the cells are responsible for storing cholesterol—essential for testosterone synthesis—and contain specialized cellular machinery for producing steroid hormones, making them a favorable target for infection. 4/ 
The research was conducted using transgenic mice that were developed in a laboratory and expressed the viral receptor ACE2. When infected, they develop COVID-19 in a similar way to humans, which allows for a better understanding of the mechanism used by the virus.
The researchers observed that both in the transgenic mouse testicle and in the human testicle, there was an intense concentration of ACE2 in the same cell types.
The result therefore validates the model used in the study and confirms that the testicle is a target organ for SARS-CoV-2, 5/
The researchers observed that both in the transgenic mouse testicle and in the human testicle, there was an intense concentration of ACE2 in the same cell types.
The result therefore validates the model used in the study and confirms that the testicle is a target organ for SARS-CoV-2, 5/
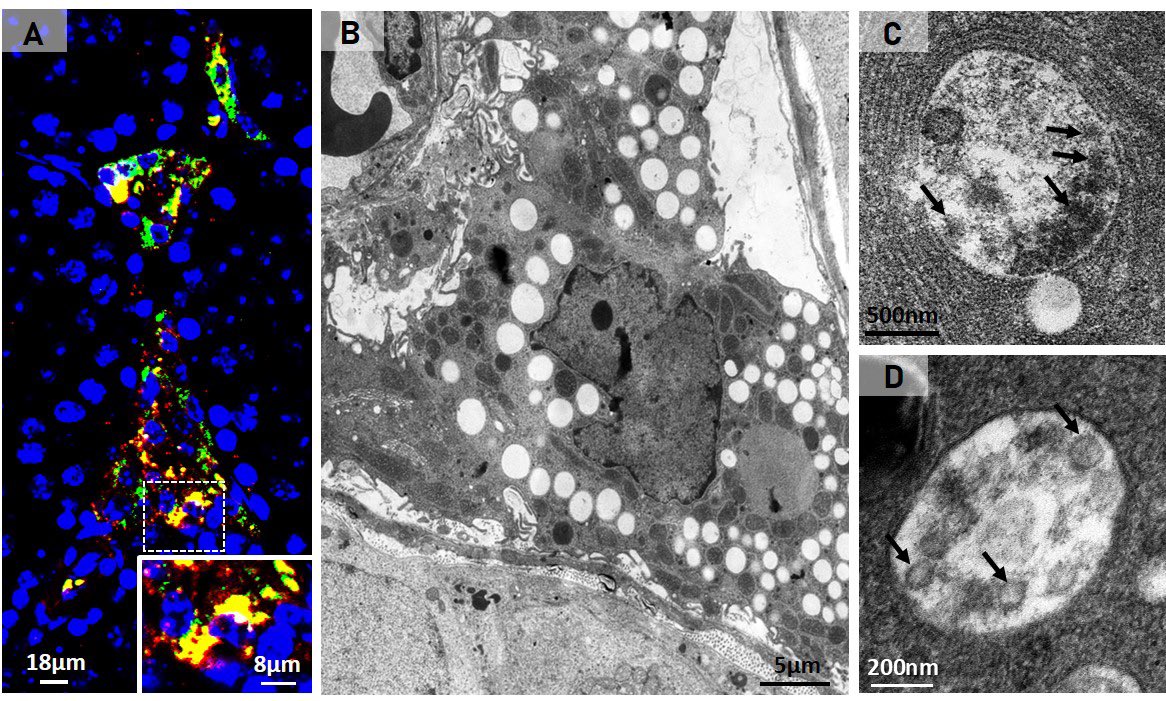
The researchers found that SARS-CoV-2 can alter the lipid metabolism of Leydig cells. This occurs because the virus uses the cholesterol stored by the cell for testosterone production to replicate itself.
Thus, despite the low testosterone levels in infected Leydig cells, they were full of lipids because the virus also induced an increase in cholesterol internalization for its own replication and formation. 6/
Thus, despite the low testosterone levels in infected Leydig cells, they were full of lipids because the virus also induced an increase in cholesterol internalization for its own replication and formation. 6/
The study also observed changes in the functional profile of Leydig cells. After being infected by the virus, they ceased producing steroid hormones from cholesterol and took on an immunological profile. 7/ 
Infection with SARS-CoV-2 also induced the Leydig cells to produce large amounts of pro-inflammatory cytokines, a process they don't normally perform. This increase in cytokines may also have interfered with testosterone production, impairing this main function, 8/ 
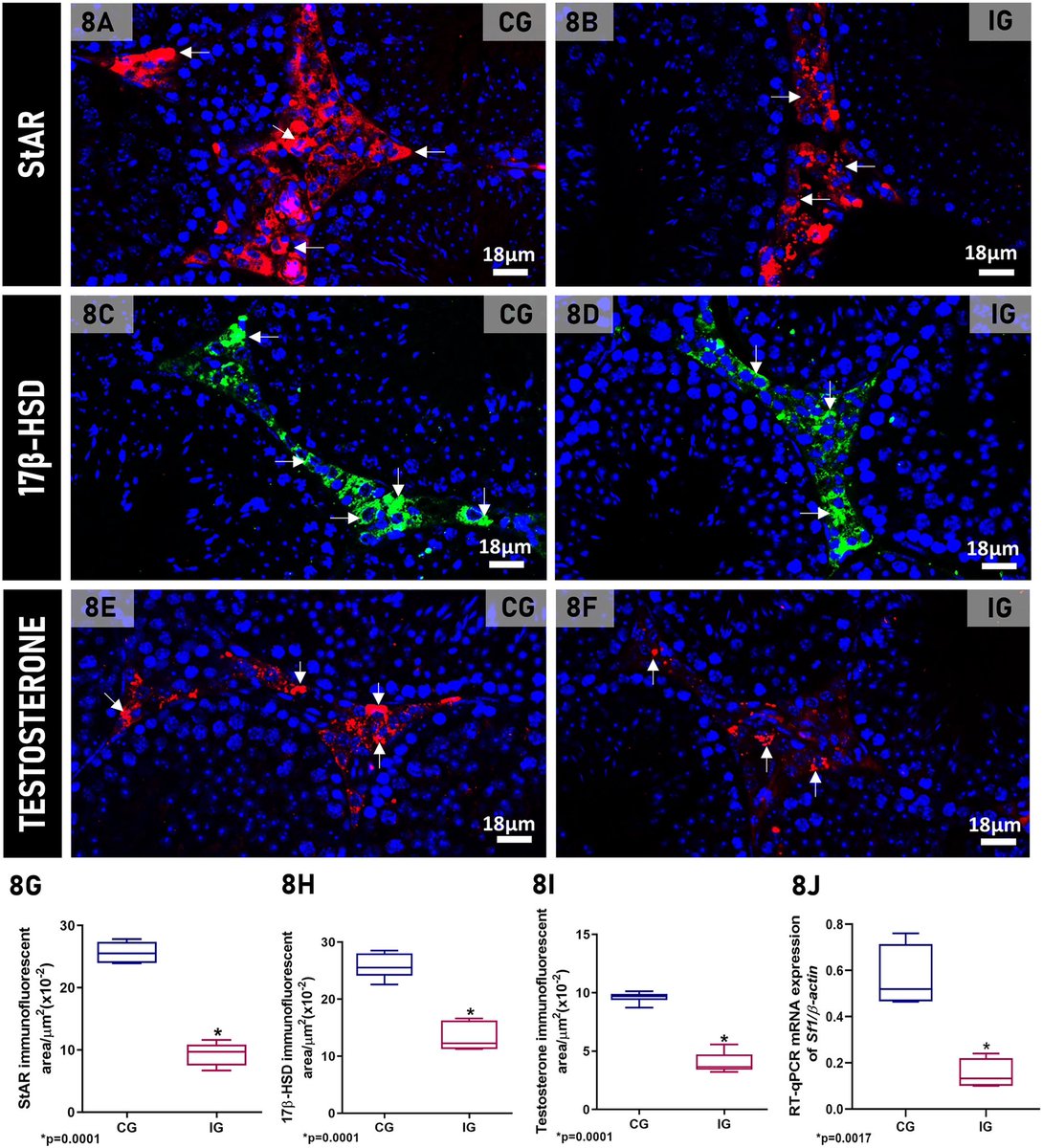
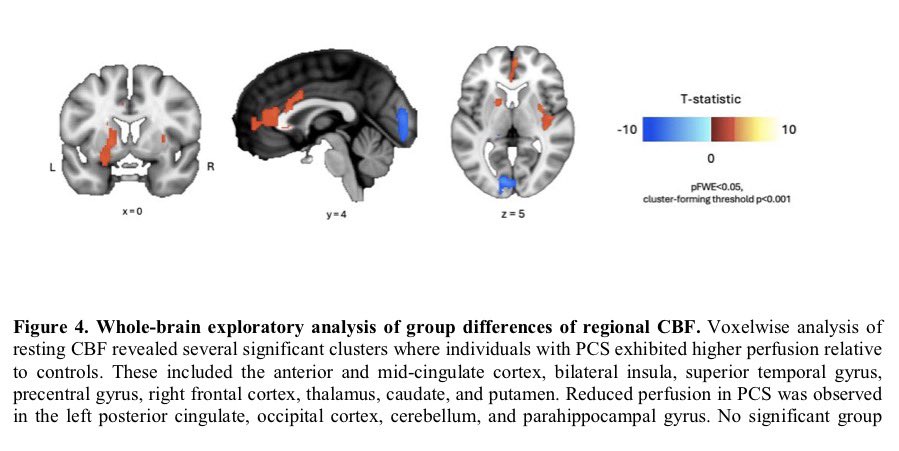
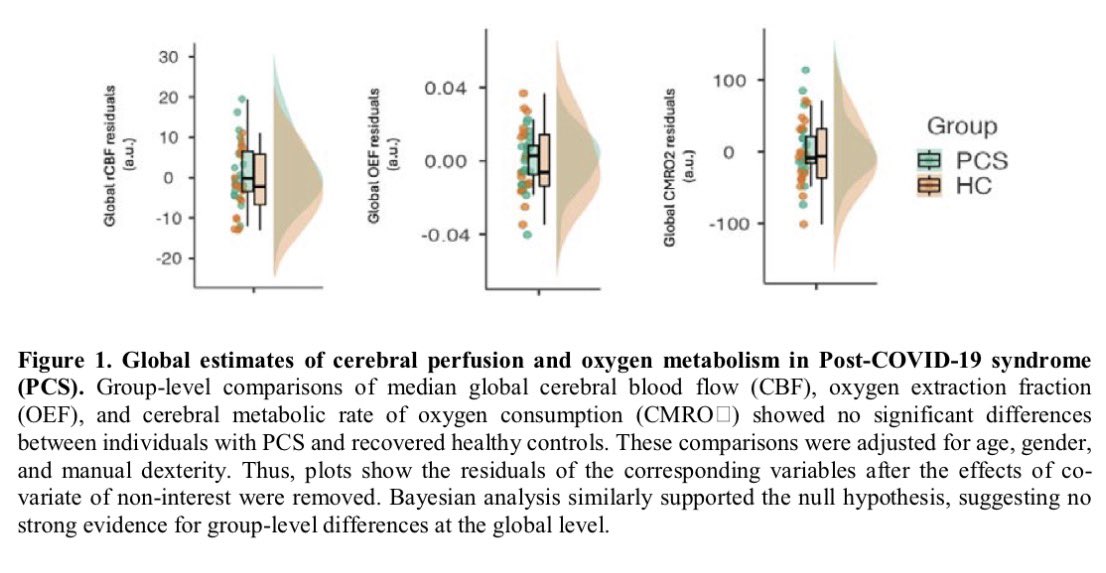
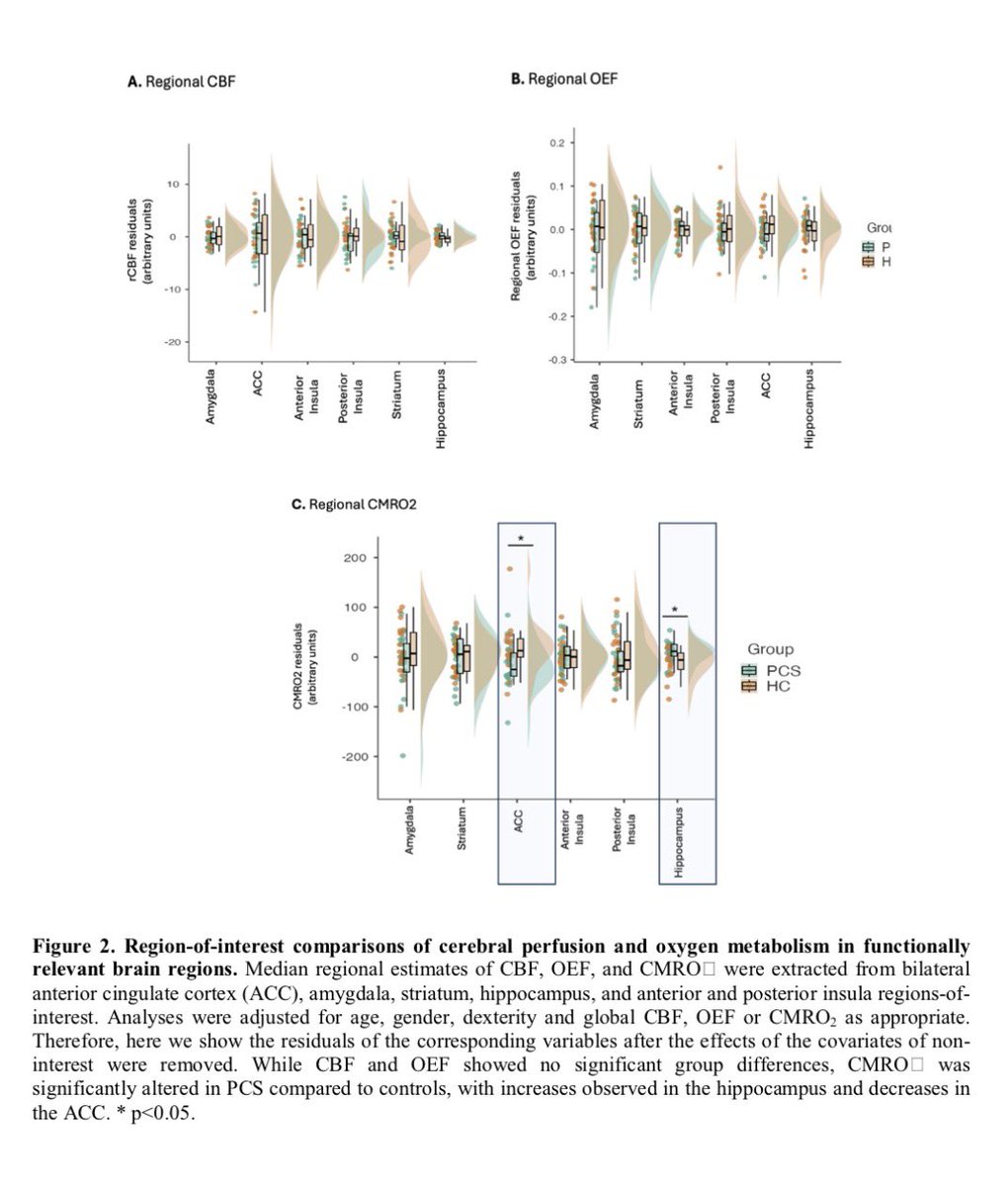
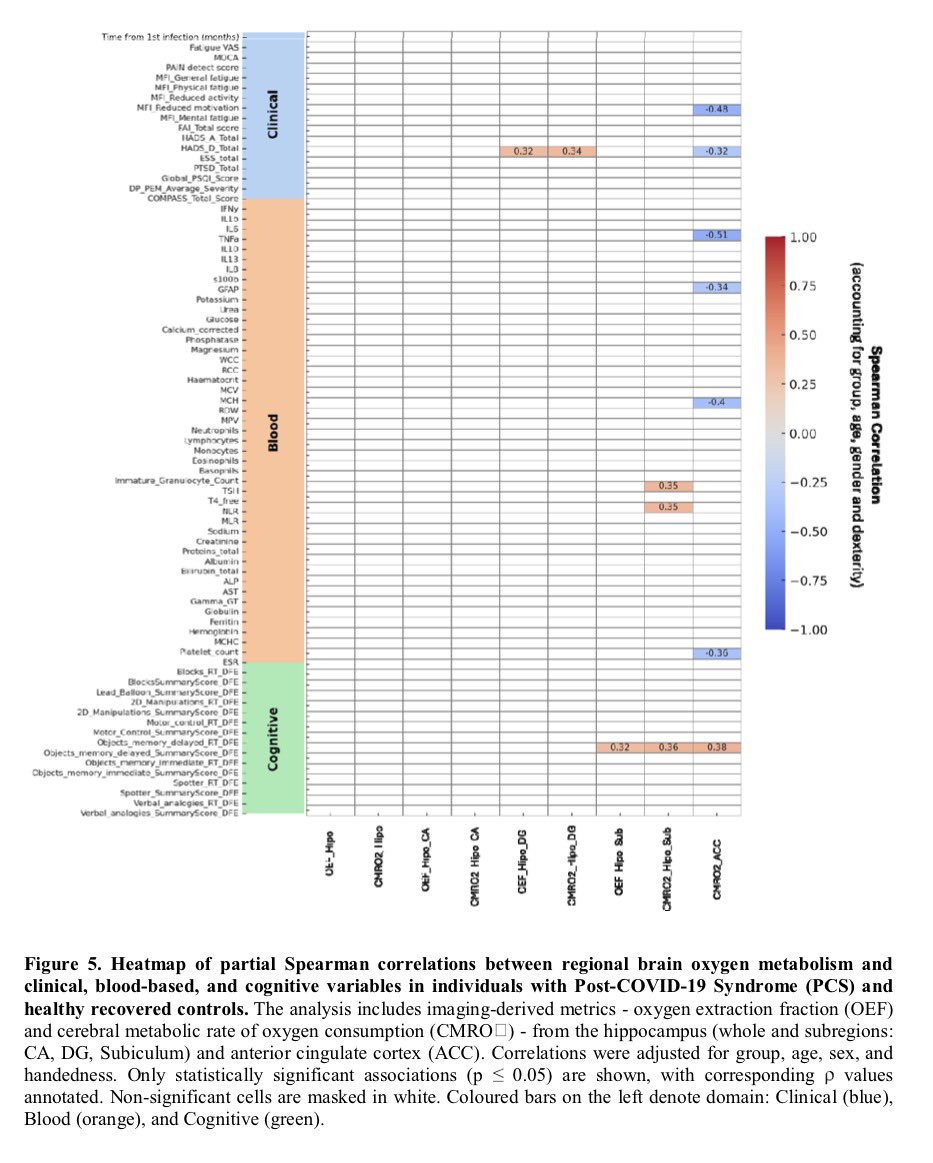
These findings advance our understanding of the cellular and molecular processes associated with testicular endocrine dysfunction caused by viral infection.
The results corroborate the clinically observed low cholesterol levels in patients with severe COVID-19 and may shed light on men's vulnerability to COVID-19 and their higher mortality rate compared to women. 9/
The results corroborate the clinically observed low cholesterol levels in patients with severe COVID-19 and may shed light on men's vulnerability to COVID-19 and their higher mortality rate compared to women. 9/
The study also paves the way for developing markers that indicate the severity of COVID-19, as well as therapies for treating the disease based on [lipid-lowering] drugs that interfere with lipid metabolism and inhibit viral action. 10/10
frontiersin.org/journals/cellu…
frontiersin.org/journals/cellu…
• • •
Missing some Tweet in this thread? You can try to
force a refresh