🔬A major new study shows clear evidence of immune overactivation, energy metabolism failure, gut issues, and worsening after exercise in ME/CFS.
Importantly - patients separated into subgroups, a focus by us @amaticahealth
Let’s break it down in simple language 🧵
Importantly - patients separated into subgroups, a focus by us @amaticahealth
Let’s break it down in simple language 🧵

@amaticahealth Researchers studied 56 people with ME/CFS and 52 healthy controls. Blood samples were taken before and after an exercise challenge that typically triggers post-exertional malaise (PEM), a core symptom of ME/CFS.
They looked at immune responses, proteins, and metabolites.
They looked at immune responses, proteins, and metabolites.
@amaticahealth Before exercise, people with ME/CFS had much stronger immune responses to bacterial and fungal mimics in lab tests. Their immune cells released more inflammatory chemicals like IL-6, TNF-alpha, and others - especially in women.
This suggests heightened immune sensitivity.
This suggests heightened immune sensitivity.
@amaticahealth Women with ME/CFS under age 45 had the strongest immune responses. Older women had higher immune markers too, though less consistently. This may be linked to estrogen levels, which help regulate the immune system.
Estrogen levels dropped with age in these patients.
Estrogen levels dropped with age in these patients.
@amaticahealth After exercise, some immune responses actually dropped in ME/CFS patients, especially in women.
This may point to immune “exhaustion” - where the system becomes overworked and unable to respond normally.
This pattern was not seen in healthy controls.
This may point to immune “exhaustion” - where the system becomes overworked and unable to respond normally.
This pattern was not seen in healthy controls.
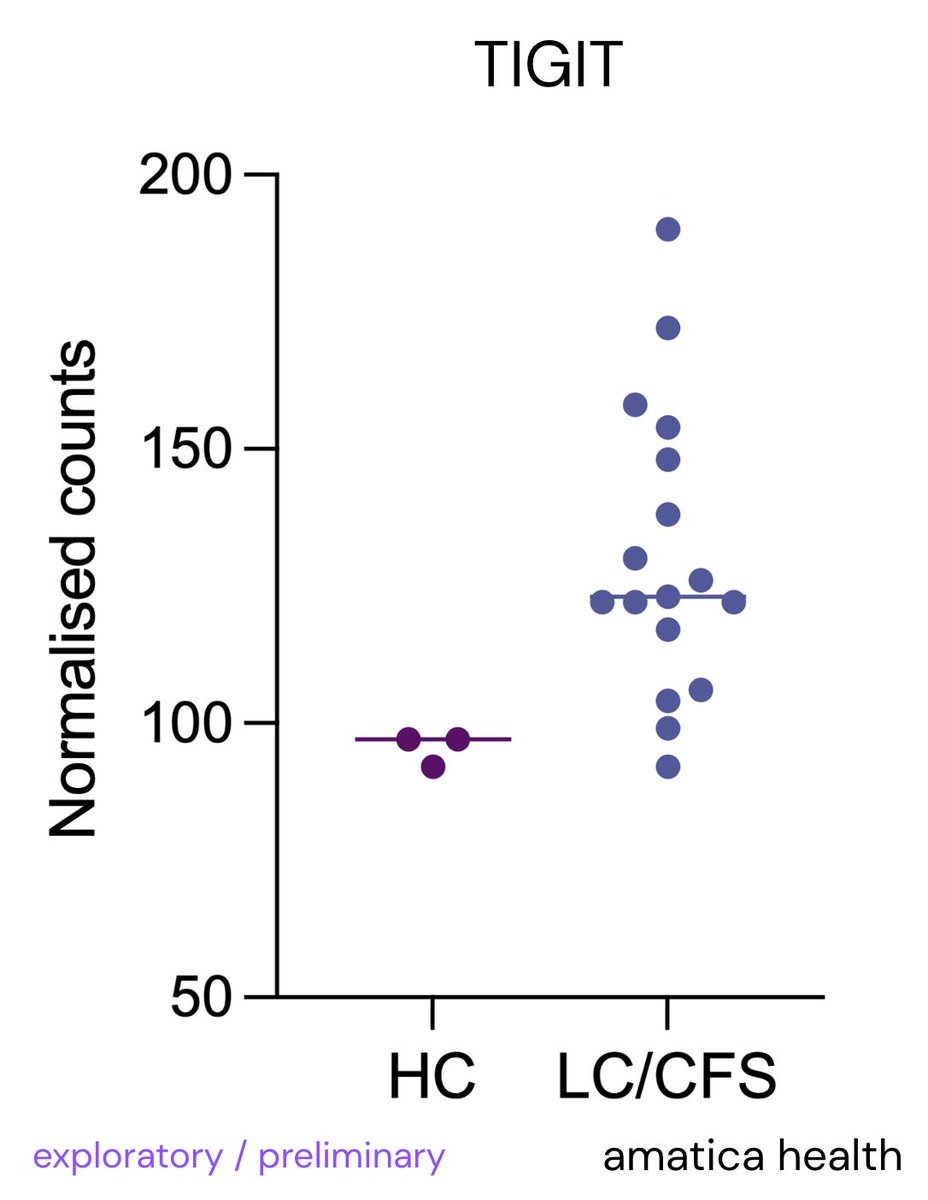
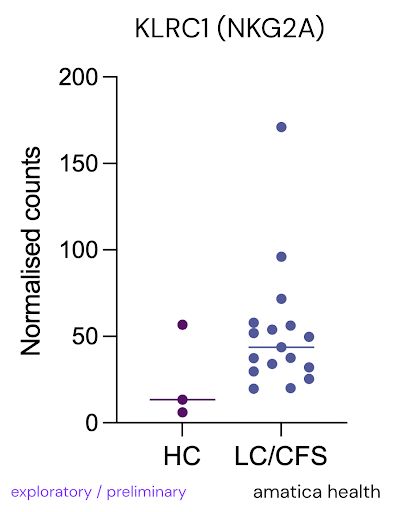
@amaticahealth We actually found T cell and NK cell immune exhaustion markers in our new RNA sequencing test @amaticahealth
TIGIT
NKG2A
We’re accepting more patient funded slots and you can join:
amaticahealth.com/me-cfs-long-co…

TIGIT
NKG2A
We’re accepting more patient funded slots and you can join:
amaticahealth.com/me-cfs-long-co…
@amaticahealth When immune cells were exposed to bacterial and viral signals (LPS and poly I:C), there were no major differences in standard tests.
But when tested more carefully, ME/CFS cells responded more at lower doses - showing increased sensitivity.
But when tested more carefully, ME/CFS cells responded more at lower doses - showing increased sensitivity.
@amaticahealth This hyper-reactivity is similar to “trained immunity,” where a past infection leads to stronger immune responses later.
The authors think ME/CFS may involve this kind of long-term immune shift, even without an active infection.
The authors think ME/CFS may involve this kind of long-term immune shift, even without an active infection.
@amaticahealth Alongside immune changes, ME/CFS patients showed clear signs of metabolic dysfunction. Their blood had abnormal levels of energy-related compounds before and after exercise.
This includes problems in the TCA cycle (the cell’s energy engine) and fat metabolism.
This includes problems in the TCA cycle (the cell’s energy engine) and fat metabolism.
@amaticahealth After exercise, healthy people showed a drop in citrate (an energy metabolite), as expected. But in ME/CFS, citrate increased.
This suggests a bottleneck in energy production - metabolism gets stuck, and energy isn’t made efficiently.
This suggests a bottleneck in energy production - metabolism gets stuck, and energy isn’t made efficiently.
@amaticahealth Another key marker: GDF15, a stress signal from cells. It was significantly higher in ME/CFS after exercise. GDF15 is linked to mitochondrial stress & exercise intolerance.
You can test your GDF15 here - we also found high levels in a subgroup:
amaticahealth.com/me-cfs-long-co…
You can test your GDF15 here - we also found high levels in a subgroup:
amaticahealth.com/me-cfs-long-co…
@amaticahealth ME/CFS patients also had higher levels of triglycerides (fats) in their blood.
At the same time, they had lower levels of carnitine and acylcarnitines - compounds needed to burn fat for energy.
This points to a failure in fat metabolism.
At the same time, they had lower levels of carnitine and acylcarnitines - compounds needed to burn fat for energy.
This points to a failure in fat metabolism.
@amaticahealth The fat-burning problem was worse after exercise.
In ME/CFS, carnitine dropped even more, and fatty acids built up. One compound called 12,13-diHOME, which helps cells take up fat, was low before exercise and abnormally high after.
This pattern was not seen in controls.
In ME/CFS, carnitine dropped even more, and fatty acids built up. One compound called 12,13-diHOME, which helps cells take up fat, was low before exercise and abnormally high after.
This pattern was not seen in controls.
@amaticahealth Gut health was disrupted in ME/CFS. Citrulline (a gut health marker) was low, while glucuronic acid (linked to poor detox) was high.
After exercise, bacterial toxins like DAP cleared in controls but not in ME/CFS - suggesting ongoing gut leak and microbial translocation.
After exercise, bacterial toxins like DAP cleared in controls but not in ME/CFS - suggesting ongoing gut leak and microbial translocation.
@amaticahealth After exercise, ME/CFS patients had higher activity in the immune system’s complement pathway.
Proteins like C1r, CFHR4, and S100A8 rose - linked to inflammation, fatigue, and brain dysfunction.
Calcium signaling, key to immune and brain function, was also disrupted.
Proteins like C1r, CFHR4, and S100A8 rose - linked to inflammation, fatigue, and brain dysfunction.
Calcium signaling, key to immune and brain function, was also disrupted.
@amaticahealth Tryptophan metabolism was off too. Normally, tryptophan helps make serotonin (a brain chemical) or gets broken down into kynurenine (KYN), which has immune and brain effects.
In ME/CFS, exercise pushed more tryptophan toward KYN and less toward serotonin.
In ME/CFS, exercise pushed more tryptophan toward KYN and less toward serotonin.
@amaticahealth At the same time, a protective compound called kynurenic acid (KYNA) decreased.
This shift suggests a move toward neurotoxic metabolites after exercise - which could help explain the “brain fog” in PEM.
This shift suggests a move toward neurotoxic metabolites after exercise - which could help explain the “brain fog” in PEM.
Brain-related proteins were altered in ME/CFS. CNTN4, EPHA4 (linked to memory and plasticity) were low at rest. After exercise, NRXN1 (nerve signaling) dropped.
The urea cycle was also disrupted, and oxidative stress markers like AOC2, CUTC, and MAPK6 rose post-exercise - signaling cell stress.
The urea cycle was also disrupted, and oxidative stress markers like AOC2, CUTC, and MAPK6 rose post-exercise - signaling cell stress.
@amaticahealth These molecular changes matched symptom severity.
Patients with higher GDF15 had worse fatigue scores.
Those with lower 12,13-diHOME had more physical fatigue and reduced activity.
Inflammation markers like S100A8 also tracked with fatigue and motivation loss.
Patients with higher GDF15 had worse fatigue scores.
Those with lower 12,13-diHOME had more physical fatigue and reduced activity.
Inflammation markers like S100A8 also tracked with fatigue and motivation loss.
@amaticahealth Subgroup analysis revealed differences by sex, age, and illness duration:
Women had stronger immune responses
Older women had lower estrogen, which may reduce immune control
Long-duration patients had worse gut and metabolic markers
Women had stronger immune responses
Older women had lower estrogen, which may reduce immune control
Long-duration patients had worse gut and metabolic markers
@amaticahealth Patients sick for >3 years had more extreme abnormalities, including:
- Lower citrulline (gut health)
- Higher inflammation
-More TCA and urea cycle disruption
- Greater shifts in tryptophan metabolism
- Lower citrulline (gut health)
- Higher inflammation
-More TCA and urea cycle disruption
- Greater shifts in tryptophan metabolism
@amaticahealth So what does this all mean?
ME/CFS involves:
- A hypersensitive, overactive immune system
- A failure to produce energy efficiently
- Gut barrier problems and microbial translocation
- A system that breaks down further after exercise
ME/CFS involves:
- A hypersensitive, overactive immune system
- A failure to produce energy efficiently
- Gut barrier problems and microbial translocation
- A system that breaks down further after exercise
@amaticahealth This study shows ME/CFS is a complex illness involving immune, metabolic, and gut-brain interactions.
Test GDF15 + other markers above here to help validate:
amaticahealth.com
Full paper:
nature.com/articles/s4432…
Test GDF15 + other markers above here to help validate:
amaticahealth.com
Full paper:
nature.com/articles/s4432…
• • •
Missing some Tweet in this thread? You can try to
force a refresh