
Aerospace engineer by training, ME/CFS & Long COVID patient researcher, cofounder @amaticahealth. DMs open, rarely check follows - https://t.co/BvmsOvch0p
3 subscribers
How to get URL link on X (Twitter) App

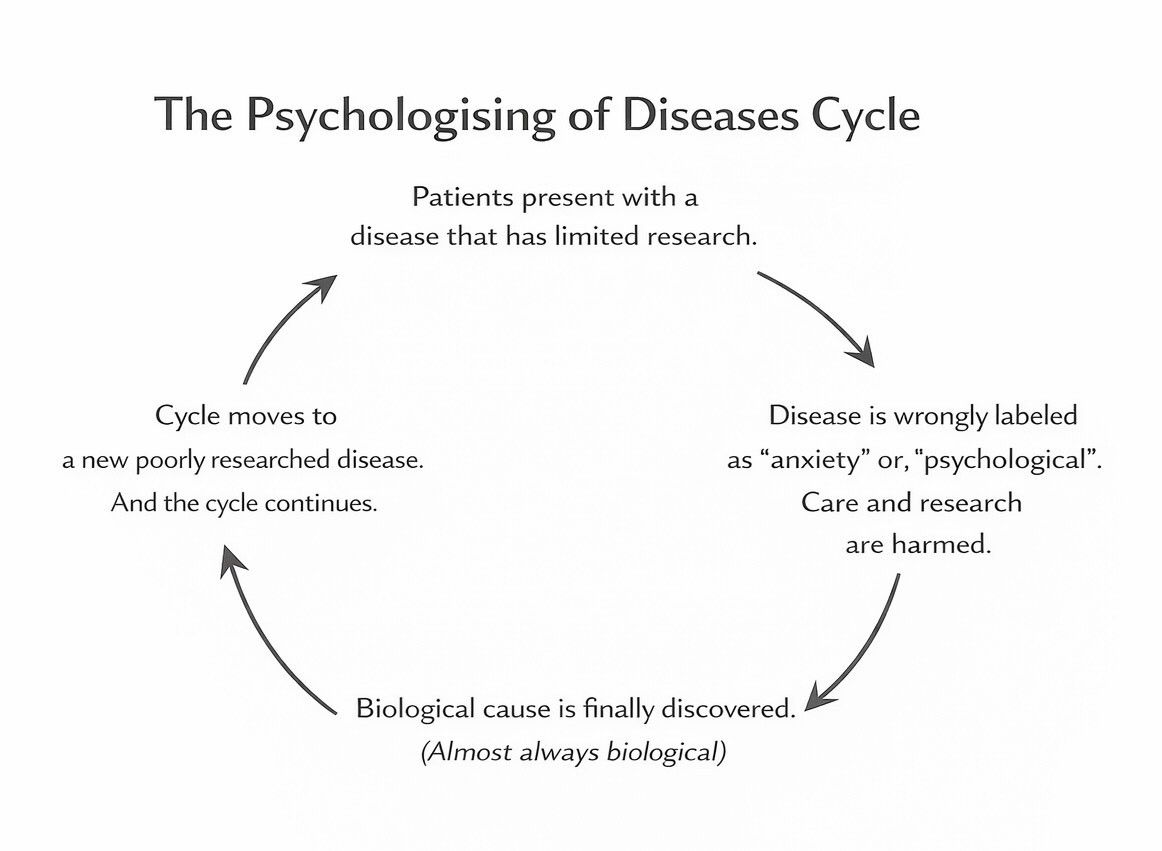
 This pattern repeats:
This pattern repeats: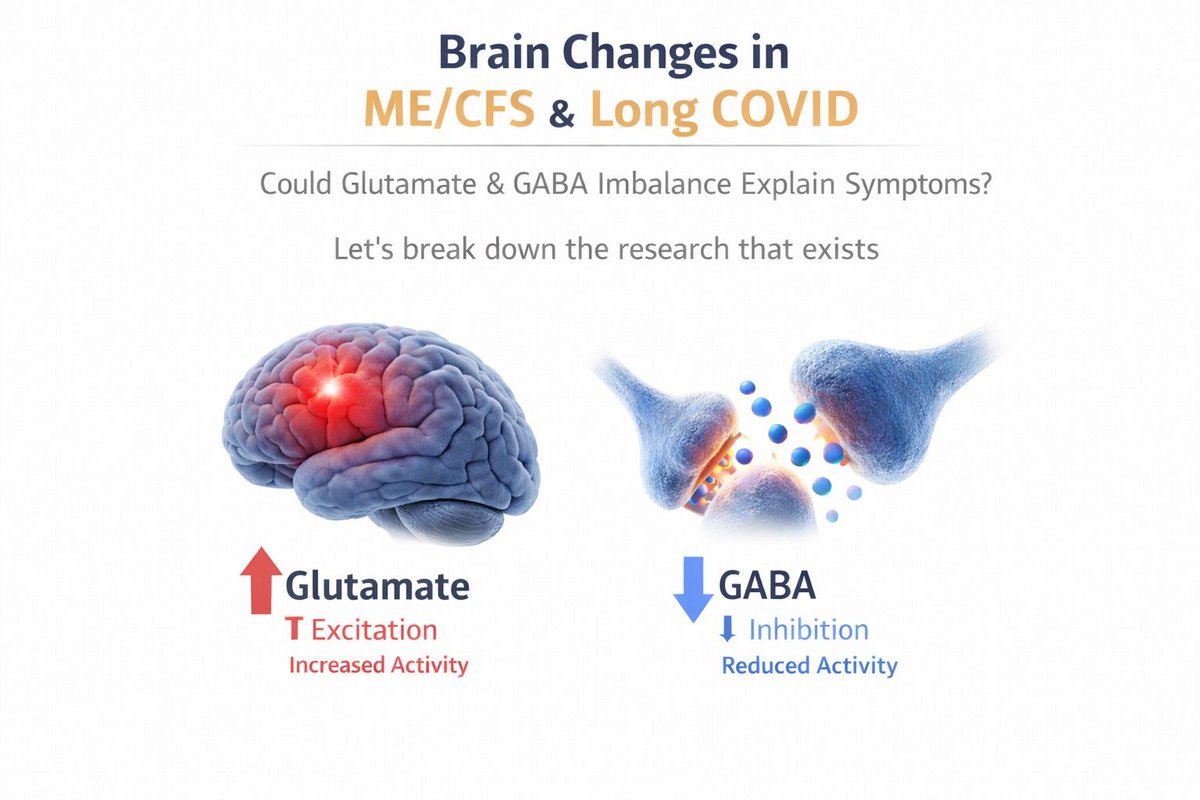
 Glutamate is a main brain signal that increases nerve cell activity.
Glutamate is a main brain signal that increases nerve cell activity. 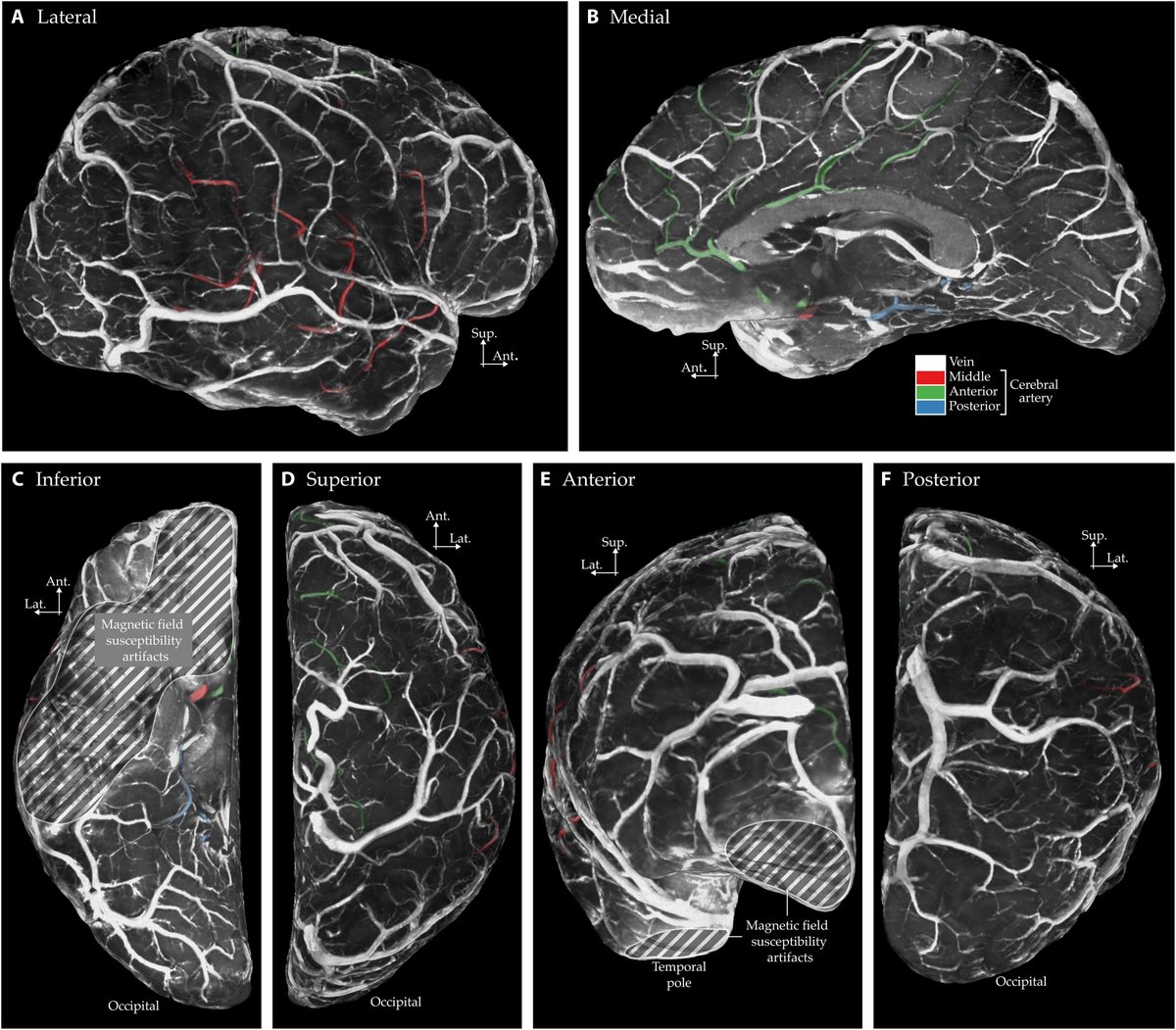
 The study used a very strong MRI scanner called 7-Tesla.
The study used a very strong MRI scanner called 7-Tesla. 
 Long COVID genetics
Long COVID genetics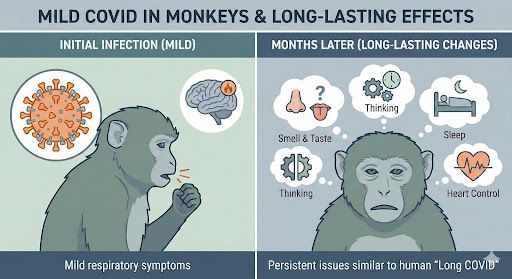
 10 adult rhesus macaques (5 female, 5 male) were infected with the early Wuhan strain. Illness was mild.
10 adult rhesus macaques (5 female, 5 male) were infected with the early Wuhan strain. Illness was mild. 
 @amaticahealth 302 trial participants had blood taken on day 1 and day 8.
@amaticahealth 302 trial participants had blood taken on day 1 and day 8. 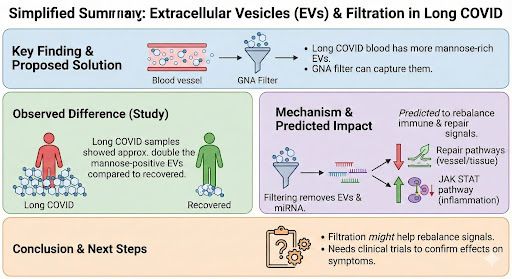
 EVs are tiny bubbles released by cells. They carry proteins and genetic material.
EVs are tiny bubbles released by cells. They carry proteins and genetic material. 
 EBV infects almost everyone, but only some people develop lupus. This study looked at how EBV behaves inside B cells in SLE compared to healthy people.
EBV infects almost everyone, but only some people develop lupus. This study looked at how EBV behaves inside B cells in SLE compared to healthy people.
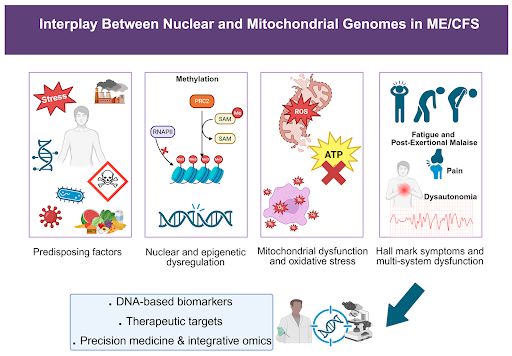
 Family and twin studies show ME/CFS is partly genetic. Identical twins share the illness much more often than non-identical ones, with heritability around 50%.
Family and twin studies show ME/CFS is partly genetic. Identical twins share the illness much more often than non-identical ones, with heritability around 50%.