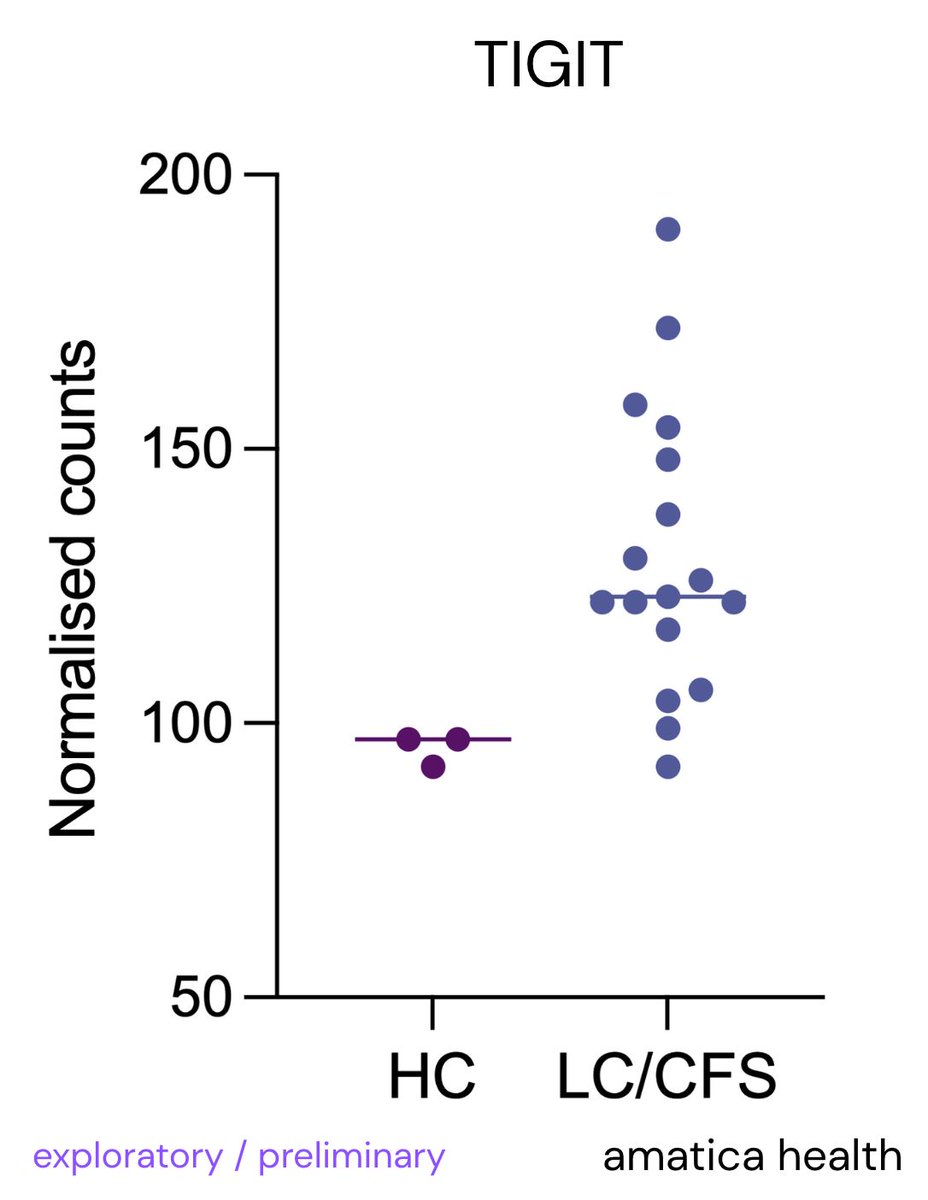
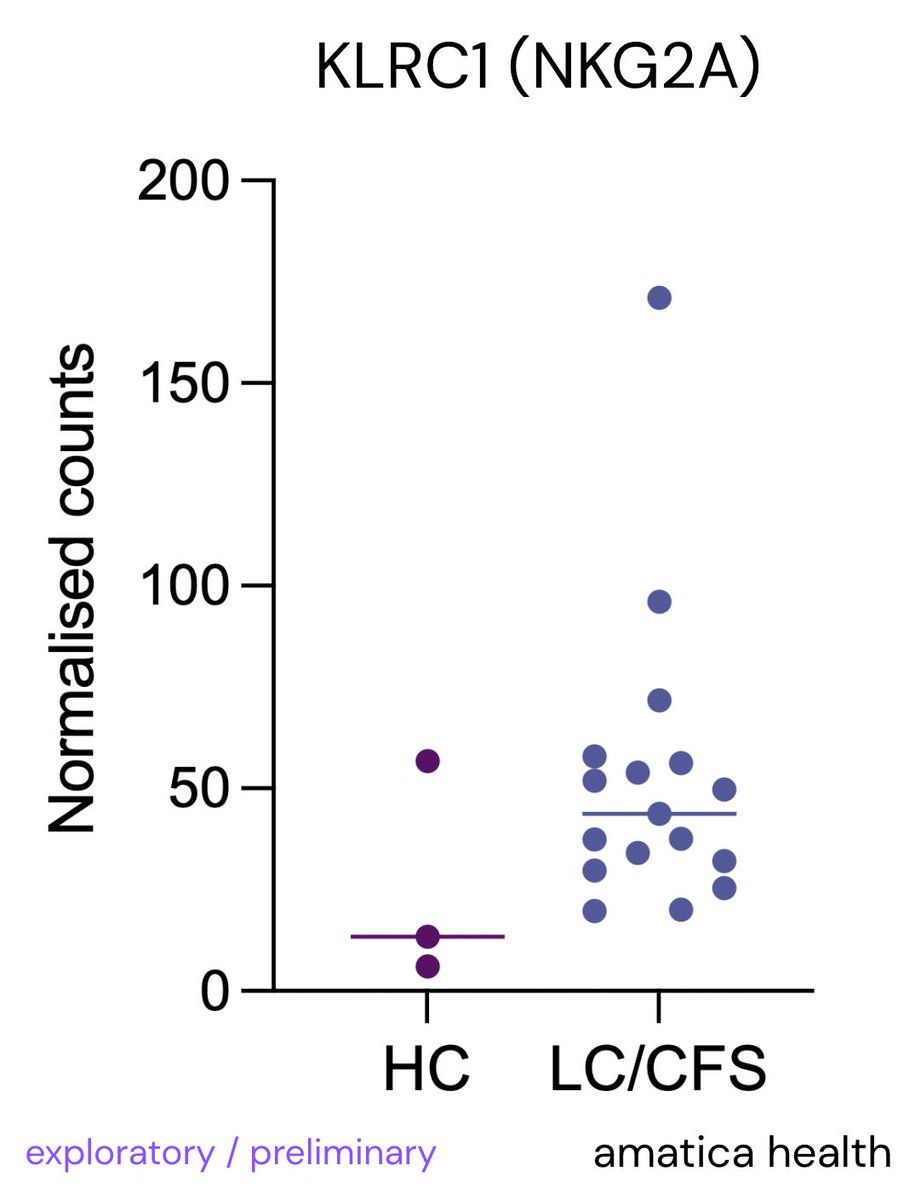
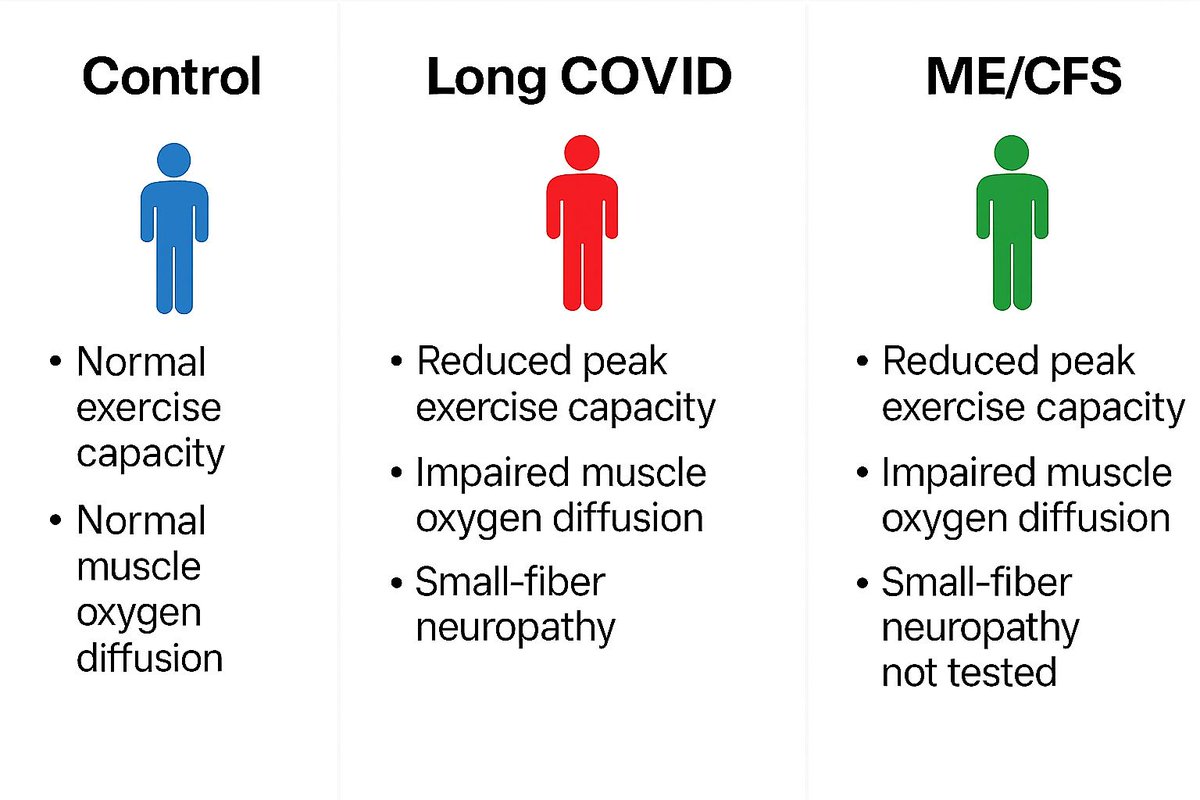
Leaky gut is when the barrier in your intestines becomes too porous, letting bacteria, toxins, or food particles slip into your bloodstream.
This thread breaks down what mRNAs are involved in that process - in plain language and how you can test yourself:
amaticahealth.com/me-cfs-long-co…
This thread breaks down what mRNAs are involved in that process - in plain language and how you can test yourself:
amaticahealth.com/me-cfs-long-co…

Your gut lining is made up of cells tightly joined together. These junctions act like seals.
Certain genes produce proteins that hold those seals in place.
In leaky gut, those genes often turn down or off, weakening the barrier.
Certain genes produce proteins that hold those seals in place.
In leaky gut, those genes often turn down or off, weakening the barrier.
Let’s look at the main mRNAs - the messages your cells use to make proteins - that play a role in leaky gut.
There are three big categories:
- Tight junctions
- Mucus and barrier repair
- Inflammatory signaling
There are three big categories:
- Tight junctions
- Mucus and barrier repair
- Inflammatory signaling
First - what is RNA?
mRNA is a copy of a gene that conveys information from the DNA to other cell locations to make proteins.
Our new @amaticahealth test includes all RNA - 20,000 results per patient.
All RNA discussed in this thread are in the test:
amaticahealth.com/me-cfs-long-co…
mRNA is a copy of a gene that conveys information from the DNA to other cell locations to make proteins.
Our new @amaticahealth test includes all RNA - 20,000 results per patient.
All RNA discussed in this thread are in the test:
amaticahealth.com/me-cfs-long-co…
@amaticahealth Tight junction genes (that seal the gut):
- CLDN1 (Claudin-1) - goes down in leaky gut
- CLDN2 (Claudin-2) - goes up and makes pores
- OCLN (Occludin) - goes down
- TJP1 (ZO-1) - goes down
- CDH1 (E-cadherin) - goes down
- CLDN1 (Claudin-1) - goes down in leaky gut
- CLDN2 (Claudin-2) - goes up and makes pores
- OCLN (Occludin) - goes down
- TJP1 (ZO-1) - goes down
- CDH1 (E-cadherin) - goes down
@amaticahealth Why does this matter?
- CLDN1 and OCLN normally keep the cells sealed
- CLDN2 forms holes that leak ions and water
- TJP1 connects the tight junctions to the inner structure of the cell
- CDH1 helps cells stick together underneath the seal
- CLDN1 and OCLN normally keep the cells sealed
- CLDN2 forms holes that leak ions and water
- TJP1 connects the tight junctions to the inner structure of the cell
- CDH1 helps cells stick together underneath the seal
@amaticahealth When these protective mRNAs drop, the proteins they code for also drop.
That makes the gut more permeable.
The “leak” starts small but can spiral into inflammation and immune activation.
That makes the gut more permeable.
The “leak” starts small but can spiral into inflammation and immune activation.
@amaticahealth Another important player: the mucus layer.
This physical barrier stops microbes from touching the gut lining directly. It’s built mainly from a gene called MUC2.
In leaky gut:
MUC2 mRNA goes down → less mucus → microbes get too close.
This physical barrier stops microbes from touching the gut lining directly. It’s built mainly from a gene called MUC2.
In leaky gut:
MUC2 mRNA goes down → less mucus → microbes get too close.
@amaticahealth MUC2 is crucial.
Mice that don’t have this gene get spontaneous gut inflammation.
In humans with ulcerative colitis, MUC2 is often low.
Less mucus means more bacterial contact and higher risk of leakiness.
Mice that don’t have this gene get spontaneous gut inflammation.
In humans with ulcerative colitis, MUC2 is often low.
Less mucus means more bacterial contact and higher risk of leakiness.
@amaticahealth Other mRNAs help stabilize or repair the mucus barrier:
- TFF3 - co-released with mucus, helps repair
- REG3B / REG3G - antimicrobial defense
These usually rise when the gut is damaged but aren’t always enough.
- TFF3 - co-released with mucus, helps repair
- REG3B / REG3G - antimicrobial defense
These usually rise when the gut is damaged but aren’t always enough.
@amaticahealth Let’s talk inflammation, which is both a cause and consequence of leaky gut. Key mRNAs here include:
- TNF (tumor necrosis factor)
- IL1B (interleukin-1 beta)
- IL6, IFNG (gamma), and IL17A
- IL22 and IL10 (protective)
- TNF (tumor necrosis factor)
- IL1B (interleukin-1 beta)
- IL6, IFNG (gamma), and IL17A
- IL22 and IL10 (protective)
@amaticahealth What do these inflammatory genes do?
-TNF and IL1B cause tight junctions to fall apart
- IL1B blocks the production of occludin
- IFNG and TNF together cause severe leakiness
- These mRNAs are high in IBD, infections, and other gut disorders
-TNF and IL1B cause tight junctions to fall apart
- IL1B blocks the production of occludin
- IFNG and TNF together cause severe leakiness
- These mRNAs are high in IBD, infections, and other gut disorders
@amaticahealth On the other hand:
- IL22 helps repair the gut lining
- IL10 keeps inflammation under control
These are often upregulated in an attempt to fix the damage.
- IL22 helps make mucus and antimicrobial peptides.
- IL22 helps repair the gut lining
- IL10 keeps inflammation under control
These are often upregulated in an attempt to fix the damage.
- IL22 helps make mucus and antimicrobial peptides.
@amaticahealth A major gene that links inflammation to leakiness is MYLK (myosin light chain kinase).
It gets turned on by TNF and IFNG.
High MYLK → cells contract and pull junctions apart → leak increases.
It’s a key driver in many leaky gut conditions.
It gets turned on by TNF and IFNG.
High MYLK → cells contract and pull junctions apart → leak increases.
It’s a key driver in many leaky gut conditions.
@amaticahealth Another gene to know is HP, which encodes zonulin, the only known human protein that can open tight junctions on demand.
In leaky gut, zonulin is high.
It’s activated by gluten and bacteria.
Blocking it with drugs like larazotide may tighten the gut barrier.
In leaky gut, zonulin is high.
It’s activated by gluten and bacteria.
Blocking it with drugs like larazotide may tighten the gut barrier.
@amaticahealth Toll-like receptors (TLRs) are also involved. These sense bacterial molecules.
In leaky gut:
TLR4 goes up → activates inflammation → worsens permeability
Especially seen in high-fat diets and metabolic disorders.
In leaky gut:
TLR4 goes up → activates inflammation → worsens permeability
Especially seen in high-fat diets and metabolic disorders.
@amaticahealth NF-κB is a master switch that turns on inflammation.
When activated, it increases TNF, IL6, IL1B, MYLK - all of which open the gut barrier.
Blocking this pathway helps restore the gut lining in animal models.
When activated, it increases TNF, IL6, IL1B, MYLK - all of which open the gut barrier.
Blocking this pathway helps restore the gut lining in animal models.
@amaticahealth Changes in these mRNAs are seen in:
- IBD (Crohn’s, UC)
- IBS (especially post-infectious)
- Celiac disease
- High-fat diets
- Alcohol exposure
- Emulsifier and additive consumption
- IBD (Crohn’s, UC)
- IBS (especially post-infectious)
- Celiac disease
- High-fat diets
- Alcohol exposure
- Emulsifier and additive consumption
@amaticahealth mRNA patterns in leaky gut:
- Barrier genes (CLDN1, OCLN, TJP1, CDH1, MUC2) go down
- Inflammatory genes (TNF, IL1B, MYLK, TLR4, CLDN2) go up
- Repair genes (IL22, REG3, TFF3) may try to compensate
- Barrier genes (CLDN1, OCLN, TJP1, CDH1, MUC2) go down
- Inflammatory genes (TNF, IL1B, MYLK, TLR4, CLDN2) go up
- Repair genes (IL22, REG3, TFF3) may try to compensate
@amaticahealth Diet matters too.
Fiber improves barrier gene expression via short-chain fatty acids like butyrate.
Fiber-deprived mice show lower IL-18 and worse permeability.
Fermentable fiber restores junction protein mRNAs and improves gut integrity.
Fiber improves barrier gene expression via short-chain fatty acids like butyrate.
Fiber-deprived mice show lower IL-18 and worse permeability.
Fermentable fiber restores junction protein mRNAs and improves gut integrity.
@amaticahealth Understanding these mRNA shifts helps us find:
- Diagnostic markers
- Drug targets
- Dietary and probiotic therapies
It’s a promising path to treating or preventing leaky gut and its downstream effects on health.
- Diagnostic markers
- Drug targets
- Dietary and probiotic therapies
It’s a promising path to treating or preventing leaky gut and its downstream effects on health.
@amaticahealth To test all the RNA above, gaining insights into your own health - while helping ME/CFS & Long COVID research - join our test now:
amaticahealth.com/me-cfs-long-co…
amaticahealth.com/me-cfs-long-co…
• • •
Missing some Tweet in this thread? You can try to
force a refresh