Public Alert No. 030/2025.
Sale of confirmed substandard and falsified ARTEMETRIN DS and CIPROFIT 500
#NafdacALERTS
bit.ly/46bnf2s



Sale of confirmed substandard and falsified ARTEMETRIN DS and CIPROFIT 500
#NafdacALERTS
bit.ly/46bnf2s




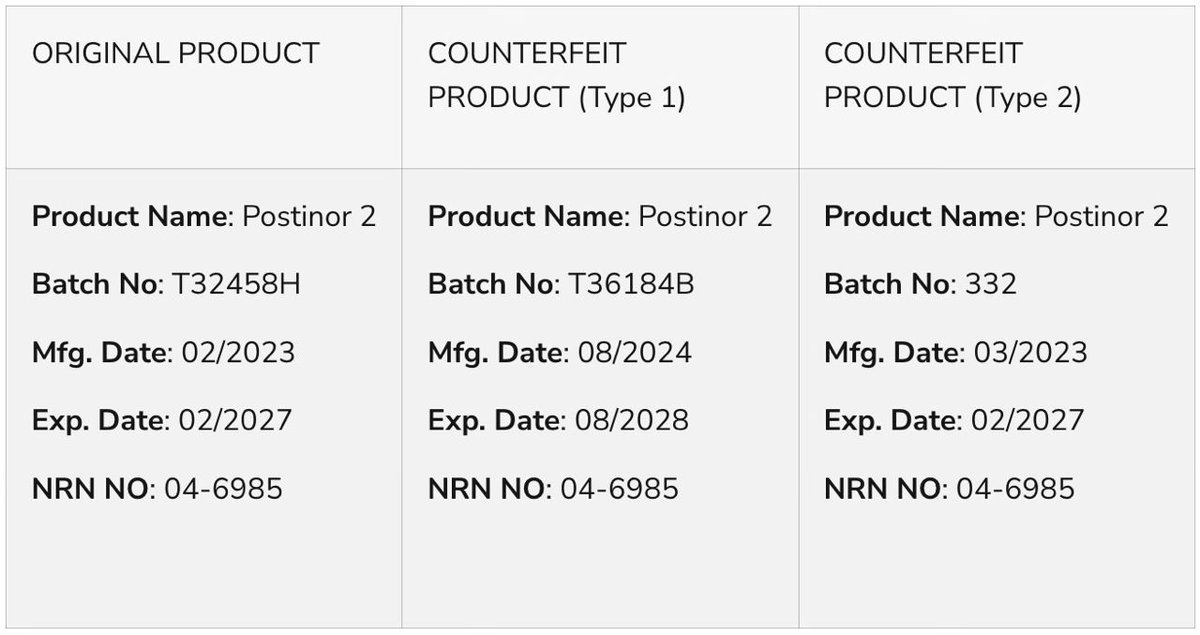
NAFDAC notifies the public of the sale of confirmed substandard and falsified ARTEMETRIN DS and CIPROFIT 500 in Nigeria.
The ARTEMETRIN DS (Artemether/Lumefantrine) tablet (80mg/480mg) is labelled manufactured by A.C. DRUGS Ltd, Plot C5/C6 Old Airport Road, Emene- Enugu State, Nigeria.
Also, the CIPROFIT 500 (Ciprofloxacin Tablet USP 500mg) is labelled manufactured by Impact Pharmaceutical Ltd, No. 33A/33B Standard Industrial Layout Emene- Enugu State, Nigeria.
The ARTEMETRIN DS (Artemether/Lumefantrine) tablet (80mg/480mg) is labelled manufactured by A.C. DRUGS Ltd, Plot C5/C6 Old Airport Road, Emene- Enugu State, Nigeria.
Also, the CIPROFIT 500 (Ciprofloxacin Tablet USP 500mg) is labelled manufactured by Impact Pharmaceutical Ltd, No. 33A/33B Standard Industrial Layout Emene- Enugu State, Nigeria.

Both products were initially subjected to Thin-Layer Chromatography (TLC), which showed indications of irregularities on both products, thereby prompting further analysis at a WHO-prequalified laboratory. The results of the HPLC assay confirmed the following:
•– ARTEMETRIN DS (Artemether/Lumefantrine) tablets (80mg/480mg) contain only 59.2% Artemether and 71.2% Lumefantrine, outside the expected 90-110% limits.
•– CIPROFIT 500 (Ciprofloxacin Tablet USP 500mg) contained only 5.7% of Ciprofloxacin, outside the expected 90-110% limits.
It is important to note that the products were purchased from a “licensed vendor and wholesaler”. The products do not exist on the NAFDAC registered products database, and all NAFDAC Registration Numbers stated on both products are false.
•– ARTEMETRIN DS (Artemether/Lumefantrine) tablets (80mg/480mg) contain only 59.2% Artemether and 71.2% Lumefantrine, outside the expected 90-110% limits.
•– CIPROFIT 500 (Ciprofloxacin Tablet USP 500mg) contained only 5.7% of Ciprofloxacin, outside the expected 90-110% limits.
It is important to note that the products were purchased from a “licensed vendor and wholesaler”. The products do not exist on the NAFDAC registered products database, and all NAFDAC Registration Numbers stated on both products are false.
If you possess any of the products listed above, stop selling or using them immediately and return your stock to the nearest NAFDAC office. If you or someone you know has used any of these products and experienced adverse reactions, we strongly advise seeking immediate medical attention from a qualified healthcare professional.
Healthcare professionals and consumers are advised to report any suspicion of substandard and falsified medicines to the nearest NAFDAC office, call 0800-162-3322, or send an email to sf.alert@nafdac.gov.ng
Healthcare professionals and consumers are advised to report any suspicion of substandard and falsified medicines to the nearest NAFDAC office, call 0800-162-3322, or send an email to sf.alert@nafdac.gov.ng
• • •
Missing some Tweet in this thread? You can try to
force a refresh