
Official account of the National Agency for Food and Drug Administration and Control, Nigeria. Follow for updates on the Agency's activities.
How to get URL link on X (Twitter) App





 NAFDAC notifies the public of the sale of confirmed substandard and falsified ARTEMETRIN DS and CIPROFIT 500 in Nigeria.
NAFDAC notifies the public of the sale of confirmed substandard and falsified ARTEMETRIN DS and CIPROFIT 500 in Nigeria.



 NAFDAC notifies the public of falsified Type 1 and Type 2 batches of POSTINOR-2 (Levonorgestrel 0.75mg) product in circulation. A report was received from the Marketing Authorisation Holder @SFHNigeria confirming that they did not import the product batch in question.
NAFDAC notifies the public of falsified Type 1 and Type 2 batches of POSTINOR-2 (Levonorgestrel 0.75mg) product in circulation. A report was received from the Marketing Authorisation Holder @SFHNigeria confirming that they did not import the product batch in question.




 NAFDAC alerts the public to the circulation of counterfeit Cowbell “Our Milk” 12g sachet milk in Nigeria. Promasidor Nigeria Ltd, the Marketing Authorisation Holder of Cowbell, stopped producing Cowbell “Our Milk” in Sept 2023 and replaced it with Cowbell “Our Creamy Goodness”.
NAFDAC alerts the public to the circulation of counterfeit Cowbell “Our Milk” 12g sachet milk in Nigeria. Promasidor Nigeria Ltd, the Marketing Authorisation Holder of Cowbell, stopped producing Cowbell “Our Milk” in Sept 2023 and replaced it with Cowbell “Our Creamy Goodness”.







 NAFDAC is notifying the public about the circulation of a confirmed falsified Cikatem (Artemether 180mg/Lumefantrine 1080mg) suspension. This product was discovered at the Coordinated Wholesale Centre (CWC) in Kano following a consumer complaint investigated by the Agency.
NAFDAC is notifying the public about the circulation of a confirmed falsified Cikatem (Artemether 180mg/Lumefantrine 1080mg) suspension. This product was discovered at the Coordinated Wholesale Centre (CWC) in Kano following a consumer complaint investigated by the Agency.





 Additionally, large consignments of banned products, including Analgin injections, diverted-free HIV antiretroviral drugs, expired medicines set for revalidation, and unregistered pharmaceuticals, were found.
Additionally, large consignments of banned products, including Analgin injections, diverted-free HIV antiretroviral drugs, expired medicines set for revalidation, and unregistered pharmaceuticals, were found.



 Acting on intelligence, a NAFDAC team raided the facility, apprehending operators offloading a 20ft container filled with unregistered carbonated drinks.
Acting on intelligence, a NAFDAC team raided the facility, apprehending operators offloading a 20ft container filled with unregistered carbonated drinks.

 NAFDAC informs healthcare providers and the public of a report of counterfeit Tecentriq injection in Nigeria.
NAFDAC informs healthcare providers and the public of a report of counterfeit Tecentriq injection in Nigeria.

 NAFDAC is informing the public of the illegal distribution and marketing of a substandard and falsified Asian Ampicillin/Cloxacillin oral suspension in Kano State.
NAFDAC is informing the public of the illegal distribution and marketing of a substandard and falsified Asian Ampicillin/Cloxacillin oral suspension in Kano State.



 In a recent enforcement operation led by NAFDAC's Investigation & Enforcement Directorate, illegal activities involving the production and distribution of various brands of alcoholic beverages were uncovered at the Trade Fair Complex in Lagos State.
In a recent enforcement operation led by NAFDAC's Investigation & Enforcement Directorate, illegal activities involving the production and distribution of various brands of alcoholic beverages were uncovered at the Trade Fair Complex in Lagos State. 





 NAFDAC is notifying the public of the sale of unregistered Seretide Accuhaler on Instagram and online media.
NAFDAC is notifying the public of the sale of unregistered Seretide Accuhaler on Instagram and online media.
 Hydroquinone cream is the standard depigmentation or skin lightening agent. Clinically, it is used to treat areas of irregular skin discolouration.
Hydroquinone cream is the standard depigmentation or skin lightening agent. Clinically, it is used to treat areas of irregular skin discolouration. 


 NAFDAC draws the attention of the public to the detection of suspected falsified Augmentin 625mg Tablets in circulation within the country with the following details and labelling lapses;
NAFDAC draws the attention of the public to the detection of suspected falsified Augmentin 625mg Tablets in circulation within the country with the following details and labelling lapses;
 The use of AstraZeneca/Oxford #COVID19Vaccine was given an approval for Emergency Use Listing (EUL) by WHO on Monday, February 15, 2021. WHO’s EUL assesses the quality, safety and efficacy of COVID-19 vaccines and is a prerequisite for COVAX Facility vaccine supply.
The use of AstraZeneca/Oxford #COVID19Vaccine was given an approval for Emergency Use Listing (EUL) by WHO on Monday, February 15, 2021. WHO’s EUL assesses the quality, safety and efficacy of COVID-19 vaccines and is a prerequisite for COVAX Facility vaccine supply.

 NAFDAC as at the time of this press release has only received application from one company for a product the company is presenting (for approval) for the treatment of the symptoms, and not for the cure of #COVID19 as a disease.
NAFDAC as at the time of this press release has only received application from one company for a product the company is presenting (for approval) for the treatment of the symptoms, and not for the cure of #COVID19 as a disease.

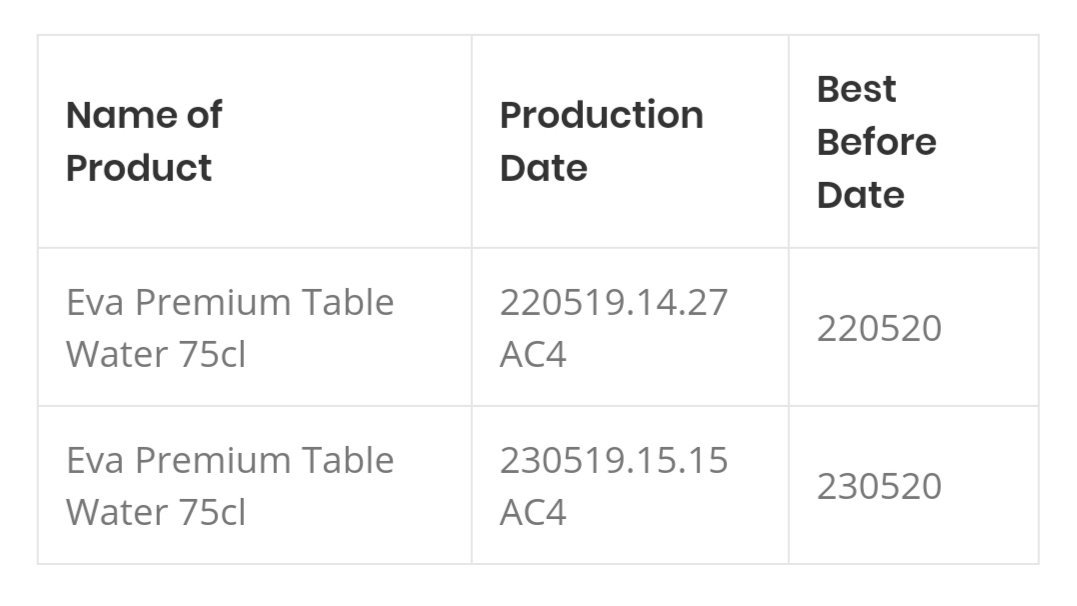
 NAFDAC has directed Nigerian Bottling Company Limited to recall Eva Premium Table Water 75cl as a precautionary step pending investigation.
NAFDAC has directed Nigerian Bottling Company Limited to recall Eva Premium Table Water 75cl as a precautionary step pending investigation.