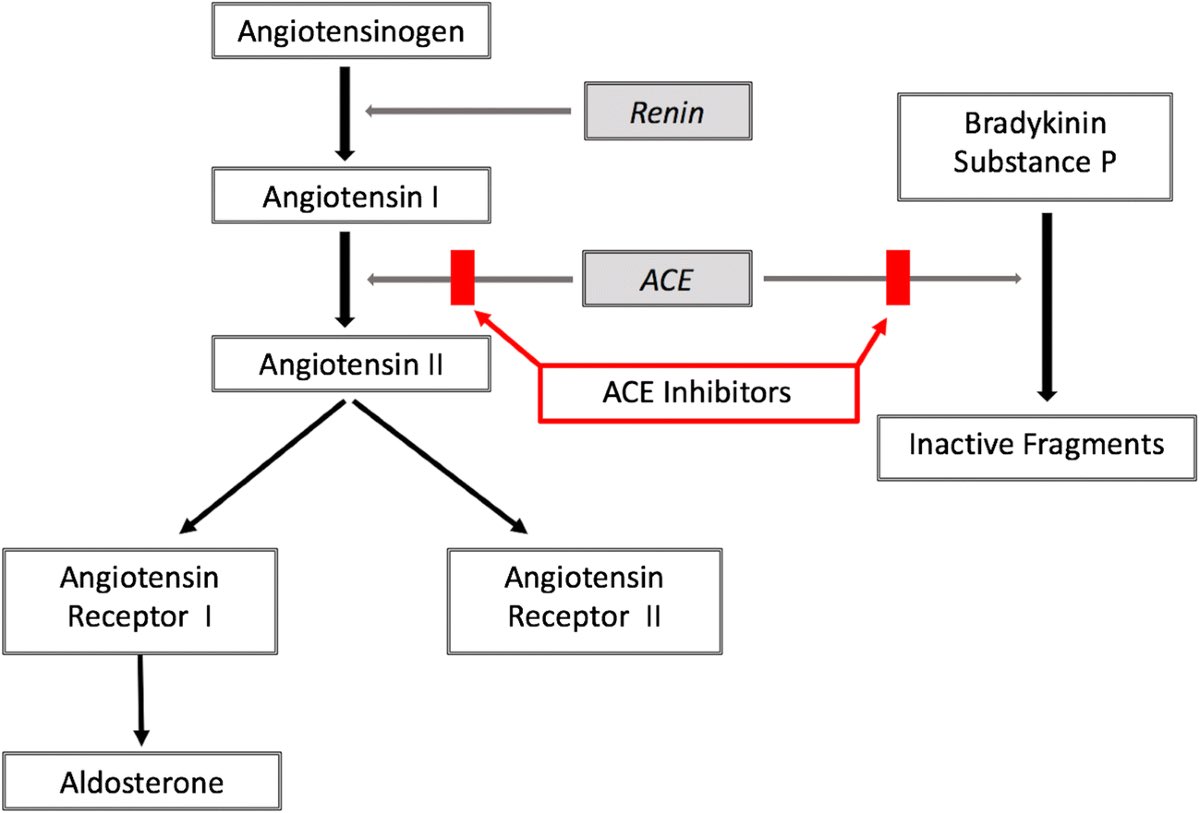
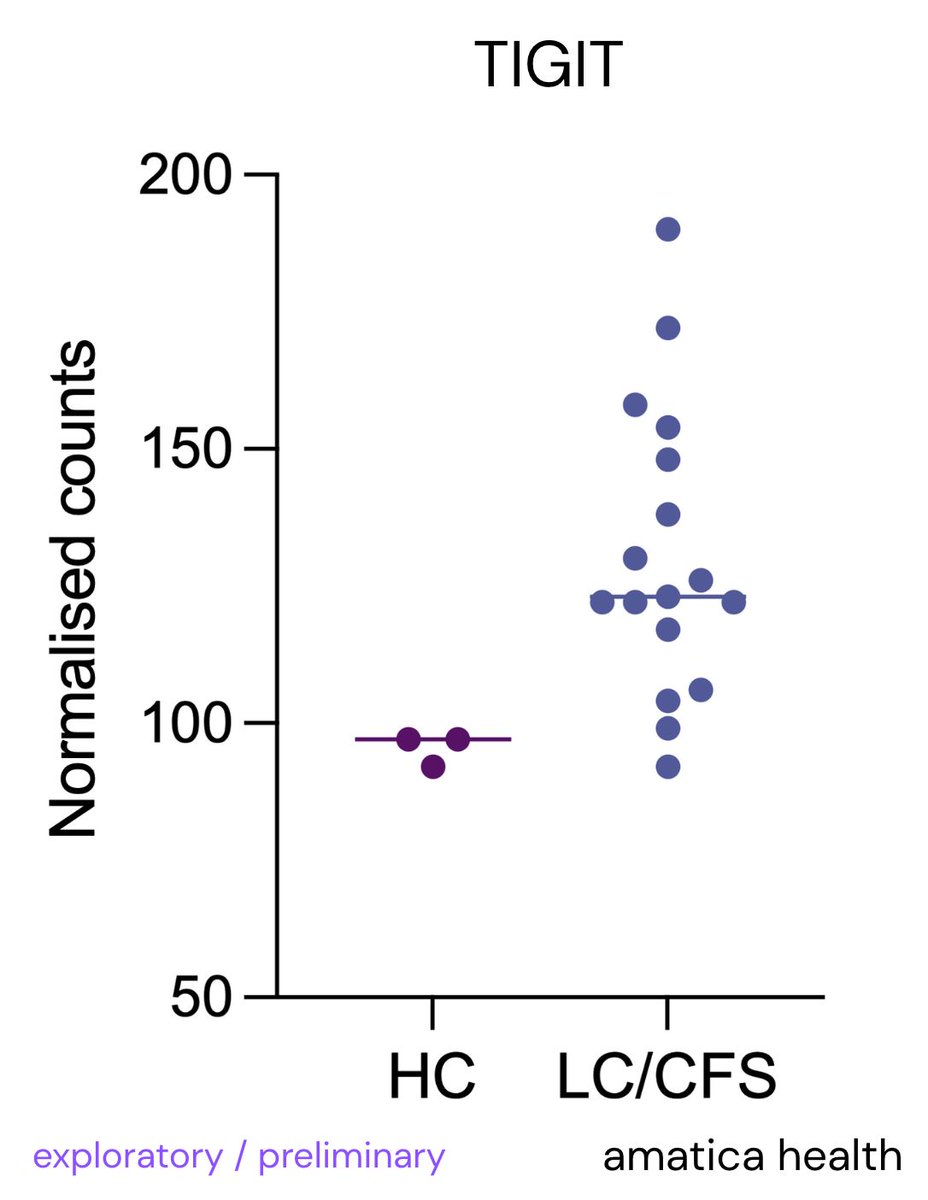
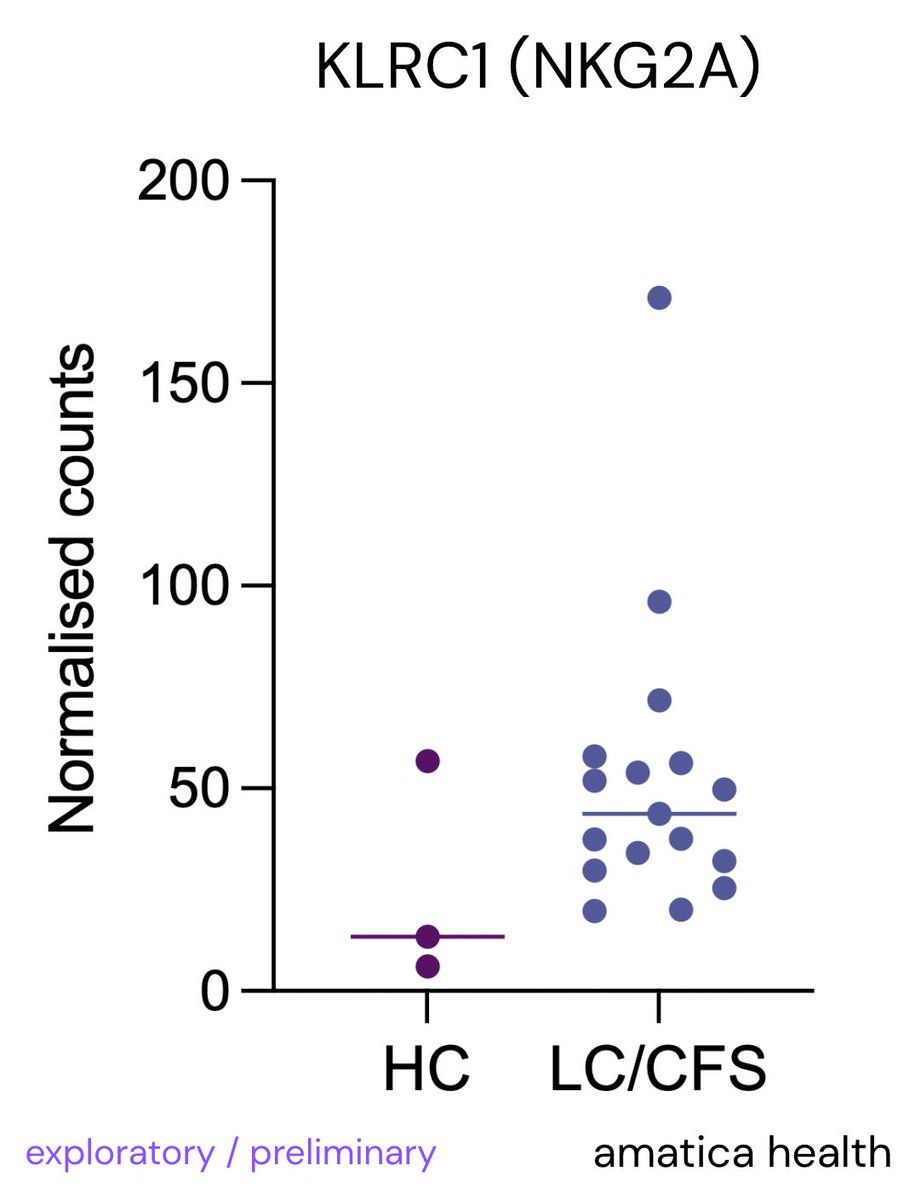
🔬Most people think mast cells are only involved in allergies or rare conditions like MCAS or mastocytosis. That’s incorrect.
Research shows mast cells are active in many diseases, including neurodegenerative, autoimmune, infectious, heart, gut, and mental health conditions🧵
Research shows mast cells are active in many diseases, including neurodegenerative, autoimmune, infectious, heart, gut, and mental health conditions🧵

Mast cells sit in tissues like the skin, gut, and around blood vessels and nerves. When triggered, they release a large mix of chemicals: histamine, tryptase, chymase, cytokines (like TNF and IL-6), prostaglandins, and more.
These chemicals affect nearby cells and tissues.
These chemicals affect nearby cells and tissues.
They don’t just respond to allergens. They’re activated by stress, infections, tissue damage, antibodies, and even certain hormones.
Once activated, they can increase inflammation, damage tissue, recruit other immune cells, and change blood vessel function.
Once activated, they can increase inflammation, damage tissue, recruit other immune cells, and change blood vessel function.
In Alzheimer’s, mast cells are found near brain areas with amyloid plaques.
They release inflammatory chemicals that damage brain cells, activate immune cells in the brain, and make the blood-brain barrier more leaky.
A mast cell drug (masitinib) slowed Alzheimer’s in trials.
They release inflammatory chemicals that damage brain cells, activate immune cells in the brain, and make the blood-brain barrier more leaky.
A mast cell drug (masitinib) slowed Alzheimer’s in trials.
In Parkinson’s, mast cells are activated in brain areas with inflammation.
They release chemicals that trigger other brain cells (like microglia and astrocytes) to release more damaging substances.
This contributes to nerve cell death over time.
They release chemicals that trigger other brain cells (like microglia and astrocytes) to release more damaging substances.
This contributes to nerve cell death over time.
In Multiple Sclerosis (MS), mast cells help immune cells cross into the brain by releasing substances that weaken the blood-brain barrier.
In animal models, removing mast cells makes MS symptoms less severe. When mast cells are restored, symptoms return.
In animal models, removing mast cells makes MS symptoms less severe. When mast cells are restored, symptoms return.
So in brain diseases, mast cells can make inflammation worse, damage protective barriers, and worsen symptoms by releasing specific chemicals like TNF, histamine, and IL-6.
In rheumatoid arthritis, mast cells are active in the joints.
They release TNF and other chemicals that cause swelling, recruit immune cells, increase blood flow, and drive tissue breakdown. They also help activate cells that destroy bone and cartilage.
They release TNF and other chemicals that cause swelling, recruit immune cells, increase blood flow, and drive tissue breakdown. They also help activate cells that destroy bone and cartilage.
In lupus, mast cells are triggered by immune complexes involving autoantibodies.
They release inflammatory chemicals in affected tissues like skin and kidneys. Some lupus patients have IgE autoantibodies, which can activate mast cells similar to an allergic reaction.
They release inflammatory chemicals in affected tissues like skin and kidneys. Some lupus patients have IgE autoantibodies, which can activate mast cells similar to an allergic reaction.
Mast cells are also found in high numbers in autoimmune thyroid disease, type 1 diabetes, and psoriasis.
In each case, their mediators contribute to inflammation, tissue damage, or fibrosis (scarring).
In each case, their mediators contribute to inflammation, tissue damage, or fibrosis (scarring).
In bacterial infections, mast cells help early on by releasing TNF and other chemicals to bring in white blood cells. They can trap bacteria directly.
But in sepsis or chronic infections, their activity can become harmful - causing leaky blood vessels, low blood pressure, and organ damage.
But in sepsis or chronic infections, their activity can become harmful - causing leaky blood vessels, low blood pressure, and organ damage.
In COVID-19, mast cells were found activated in the lungs.
Their mediators likely contributed to fluid buildup, lung injury, and the severe inflammatory response in some patients. Researchers have proposed using mast cell blockers in COVID-related lung damage.
Their mediators likely contributed to fluid buildup, lung injury, and the severe inflammatory response in some patients. Researchers have proposed using mast cell blockers in COVID-related lung damage.
In dengue and RSV, mast cells worsen symptoms by releasing chemicals that increase blood vessel leak or cause asthma-like responses.
In parasite infections, they help remove worms from the gut, but if overactive, can cause long-term inflammation or fibrosis.
In parasite infections, they help remove worms from the gut, but if overactive, can cause long-term inflammation or fibrosis.
In heart disease, mast cells are found in inflamed arteries. They help start the plaque process by allowing LDL (“bad” cholesterol) and immune cells into the vessel wall.
They release enzymes that weaken plaque walls, making heart attacks more likely.
They release enzymes that weaken plaque walls, making heart attacks more likely.
In arrhythmias, mast cell activity affects how electrical signals move through the heart.
They help create the fibrotic tissue that makes certain types of rhythm problems more likely, especially in atrial fibrillation.
They help create the fibrotic tissue that makes certain types of rhythm problems more likely, especially in atrial fibrillation.
In Crohn’s disease and ulcerative colitis, mast cells are increased in the gut.
They release histamine, TNF, and enzymes that make the gut lining more permeable (“leaky”), worsen inflammation, and cause pain.
They release histamine, TNF, and enzymes that make the gut lining more permeable (“leaky”), worsen inflammation, and cause pain.
In IBS, mast cells are often found close to gut nerves.
The more mast cells near nerves, the worse the pain.
They release chemicals that make nerves more sensitive to normal gut activity, causing pain, bloating, urgency, and diarrhea.
The more mast cells near nerves, the worse the pain.
They release chemicals that make nerves more sensitive to normal gut activity, causing pain, bloating, urgency, and diarrhea.
Stress triggers mast cells in the gut to release these substances. They break down tight junctions in the gut lining, allowing irritants in. Mast cell stabilizers and antihistamines have shown benefit in some IBS cases.
In depression, anxiety, and PTSD, mast cells in the brain are activated by stress.
They release IL-6, TNF, and VEGF, which cause inflammation, disrupt the blood-brain barrier, and change how brain cells communicate.
They release IL-6, TNF, and VEGF, which cause inflammation, disrupt the blood-brain barrier, and change how brain cells communicate.
Chronic mast cell activation in the brain is linked to changes in serotonin pathways and worsening of mood symptoms. Elevated inflammation markers in depression may be partly driven by mast cells.
In migraine, mast cells in the brain’s outer layers release chemicals that activate pain pathways.
This likely contributes to migraine attacks. Some mast cell blockers have shown benefit in migraine prevention.
This likely contributes to migraine attacks. Some mast cell blockers have shown benefit in migraine prevention.
In autism, some research suggests early mast cell activation may interfere with brain development.
This is still an emerging area, but findings include elevated mast cell-related markers and some reports of symptom improvement with mast cell-targeting compounds.
This is still an emerging area, but findings include elevated mast cell-related markers and some reports of symptom improvement with mast cell-targeting compounds.
Mast cells influence disease by releasing a wide mix of powerful mediators. These affect nerves, immune cells, blood vessels, connective tissue, and even brain function. Their role varies by context, but they are not just allergy cells.
In short:
mast cells are involved in many diseases beyond allergy. They contribute to inflammation, tissue damage, nerve sensitivity, immune dysregulation, fibrosis, and even mental health changes.
Reposted this one as I think it’s so important.
mast cells are involved in many diseases beyond allergy. They contribute to inflammation, tissue damage, nerve sensitivity, immune dysregulation, fibrosis, and even mental health changes.
Reposted this one as I think it’s so important.
Mast cells need to be studied more broadly and taken seriously in disease models outside of allergy.
If you’re treating or researching chronic disease, don’t ignore mast cells.
We at @amaticahealth will be reviewing mast cells related RNA - join here:
amaticahealth.com/me-cfs-long-co…
If you’re treating or researching chronic disease, don’t ignore mast cells.
We at @amaticahealth will be reviewing mast cells related RNA - join here:
amaticahealth.com/me-cfs-long-co…
• • •
Missing some Tweet in this thread? You can try to
force a refresh