🔬❕We found reduced ACE protein levels in the blood of people with Long COVID & ME/CFS.
This could affect blood flow, fluid balance, inflammation, & brain function - potentially relevant to symptoms like orthostatic intolerance, brain fog, & chronic pain.
Let’s break it down.
This could affect blood flow, fluid balance, inflammation, & brain function - potentially relevant to symptoms like orthostatic intolerance, brain fog, & chronic pain.
Let’s break it down.

ACE (angiotensin-converting enzyme) plays a key role in blood pressure, fluid balance, and inflammation.
It converts angiotensin I to angiotensin II, and also breaks down bradykinin and substance P - both of which affect pain, blood vessels, and immune signaling.
It converts angiotensin I to angiotensin II, and also breaks down bradykinin and substance P - both of which affect pain, blood vessels, and immune signaling.
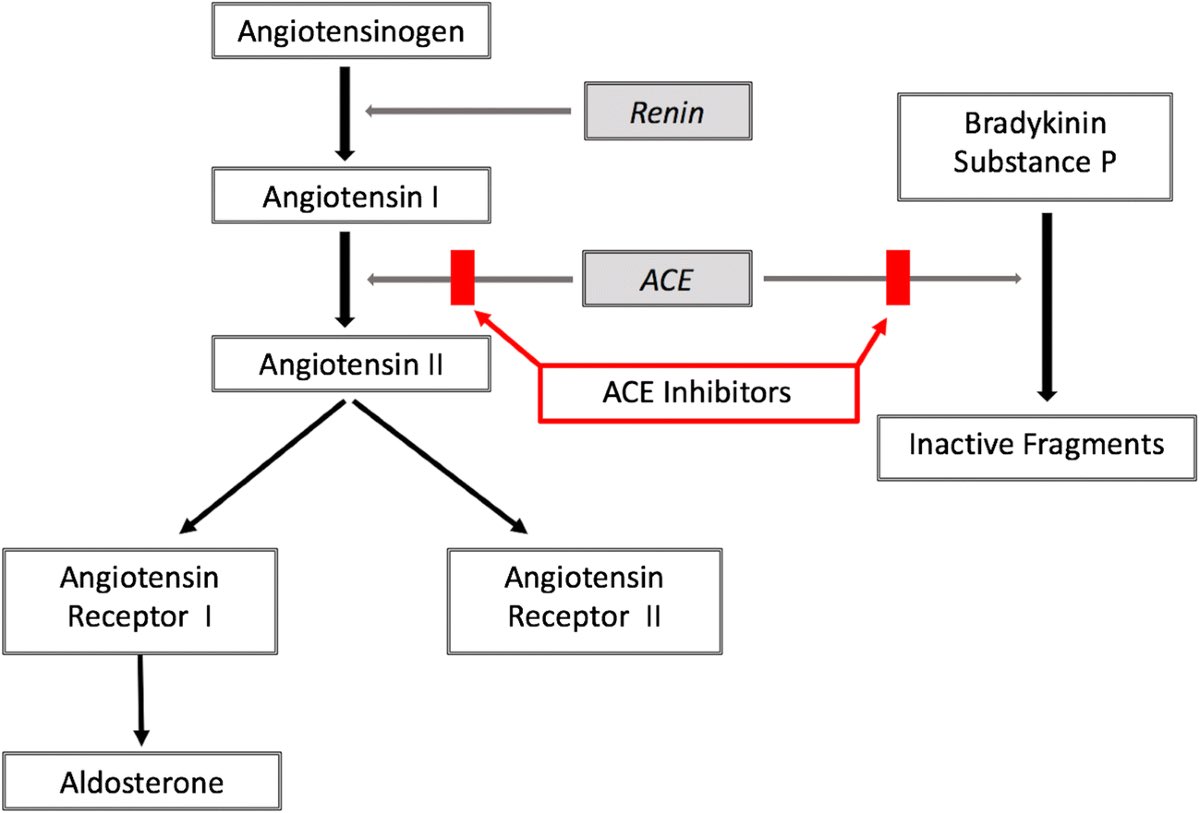
Low ACE in plasma may mean the body can’t break down bradykinin and substance P efficiently.
These molecules can cause blood vessel leakiness, low blood pressure, inflammation, pain sensitivity, and cognitive symptoms - all seen in ME/CFS and Long COVID.
These molecules can cause blood vessel leakiness, low blood pressure, inflammation, pain sensitivity, and cognitive symptoms - all seen in ME/CFS and Long COVID.
We measured ACE protein (not activity) using ELISA in platelet-rich plasma from patients with ME/CFS and Long COVID.
The trend was consistent across two independent runs.
The trend was consistent across two independent runs.
ACE is normally found in the blood as a soluble protein shed from cells that line blood vessels.
If those cells are damaged or not releasing ACE properly, circulating ACE levels can drop.
If those cells are damaged or not releasing ACE properly, circulating ACE levels can drop.
It’s also possible that platelets are holding on to ACE or failing to release it.
Platelets have their own local renin-angiotensin system.
If their behavior is altered in these illnesses, it could affect how much ACE ends up in the plasma.
Platelets have their own local renin-angiotensin system.
If their behavior is altered in these illnesses, it could affect how much ACE ends up in the plasma.
We also know from past research that serum ACE activity (a related but different measure) is often low in acute COVID-19 and is linked to worse outcomes, higher inflammation, and poor immune responses.
Reduced ACE levels in Long COVID and ME/CFS could be a lingering effect of viral or immune injury to the vascular system.
Or it could reflect long-term changes in gene expression, protein shedding, or clearance.
Or it could reflect long-term changes in gene expression, protein shedding, or clearance.
What happens downstream when ACE is low?
Bradykinin and substance P may stick around longer, contributing to effects that include some common in both conditions:
- Low blood pressure
- Dizziness when standing
- Brain fog
- Pain
- Edema
- Inflammation
Bradykinin and substance P may stick around longer, contributing to effects that include some common in both conditions:
- Low blood pressure
- Dizziness when standing
- Brain fog
- Pain
- Edema
- Inflammation
ACE also helps regulate the renin-angiotensin-aldosterone system (RAAS), which controls salt, water, and blood pressure.
If ACE is low, angiotensin II and aldosterone may also be low.
This is because ACE converts AngI into AngII
If ACE is low, angiotensin II and aldosterone may also be low.
This is because ACE converts AngI into AngII
This fits into some findings in previous ME/CFS studies showing low aldosterone and renin, despite low blood volume and cardiac output.
However, the previous research shows some people with ME/CFS or Long COVID may also show high angiotensin II (Ang II).
However, the previous research shows some people with ME/CFS or Long COVID may also show high angiotensin II (Ang II).
This may seem contradictory but can happen when other enzymes (like chymase) are producing Ang II outside of the usual ACE pathway.
In that case, you may have both high Ang II and low ACE activity at the same time.
That could create a situation where blood vessels constrict (Ang II) and leak (bradykinin) - pulling the system in two directions at once.
That could create a situation where blood vessels constrict (Ang II) and leak (bradykinin) - pulling the system in two directions at once.
This could potentially explain why some patients experience fluctuations:
sometimes lightheaded and volume-depleted, other times flushed or hypertensive, sometimes both.
The body’s signaling is unbalanced across multiple pathways. But more research is needed to confirm.
sometimes lightheaded and volume-depleted, other times flushed or hypertensive, sometimes both.
The body’s signaling is unbalanced across multiple pathways. But more research is needed to confirm.
We can determine if there is a correlation between ACE and AngII when we receive the AngII results in the coming weeks.
Low ACE also affects neuropeptides.
For example, it helps clear substance P, which contributes to inflammation and amplifies pain.
If ACE is down, substance P may accumulate and contribute to fatigue, hypersensitivity, and brain fog.
For example, it helps clear substance P, which contributes to inflammation and amplifies pain.
If ACE is down, substance P may accumulate and contribute to fatigue, hypersensitivity, and brain fog.
In the central nervous system, substance P can trigger neuroinflammation, activate immune cells in the brain, and worsen cognitive symptoms.
These effects may persist in a chronic low-ACE state.
These effects may persist in a chronic low-ACE state.
Bradykinin and substance P together can increase blood-brain barrier permeability.
This may allow immune signals to enter the brain more easily and contribute to cognitive and neurological symptoms.
This may allow immune signals to enter the brain more easily and contribute to cognitive and neurological symptoms.
ACE also breaks down Ac-SDKP, a peptide involved in blood formation and fibrosis.
If ACE is low, Ac-SDKP may rise.
This could have additional effects on the immune system, inflammation, or tissue remodeling.
If ACE is low, Ac-SDKP may rise.
This could have additional effects on the immune system, inflammation, or tissue remodeling.
Altogether, a low ACE state could lead to:
- Increase bradykinin
- Increase substance P
- Altered angII levels
- Altered aldosterone
This creates a biological environment that aligns with some symptoms of ME/CFS and Long COVID.
- Increase bradykinin
- Increase substance P
- Altered angII levels
- Altered aldosterone
This creates a biological environment that aligns with some symptoms of ME/CFS and Long COVID.
These findings suggest a shift in the balance of key systems that regulate circulation, inflammation, and neuropeptides.
It could play a part in how viral illness leads to chronic symptoms in susceptible individuals.
It could play a part in how viral illness leads to chronic symptoms in susceptible individuals.
This also opens up new questions about the role of the ACE-bradykinin-substance P axis and ACE-AngII conversion in long-term post-viral illness.
Test your ACE here, alongside 30 other markers & see how you fit into the research:
amaticahealth.com/me-cfs-long-co…
amaticahealth.com/me-cfs-long-co…

Limitations:
This analysis is exploratory, with modest sample size and a borderline difference between groups. It did not meet conventional significance, and there is considerable overlap in individual values. Further work in larger cohorts will be needed to confirm whether the observed trend toward reduced ACE reflects a true biological signal.
This analysis is exploratory, with modest sample size and a borderline difference between groups. It did not meet conventional significance, and there is considerable overlap in individual values. Further work in larger cohorts will be needed to confirm whether the observed trend toward reduced ACE reflects a true biological signal.
• • •
Missing some Tweet in this thread? You can try to
force a refresh