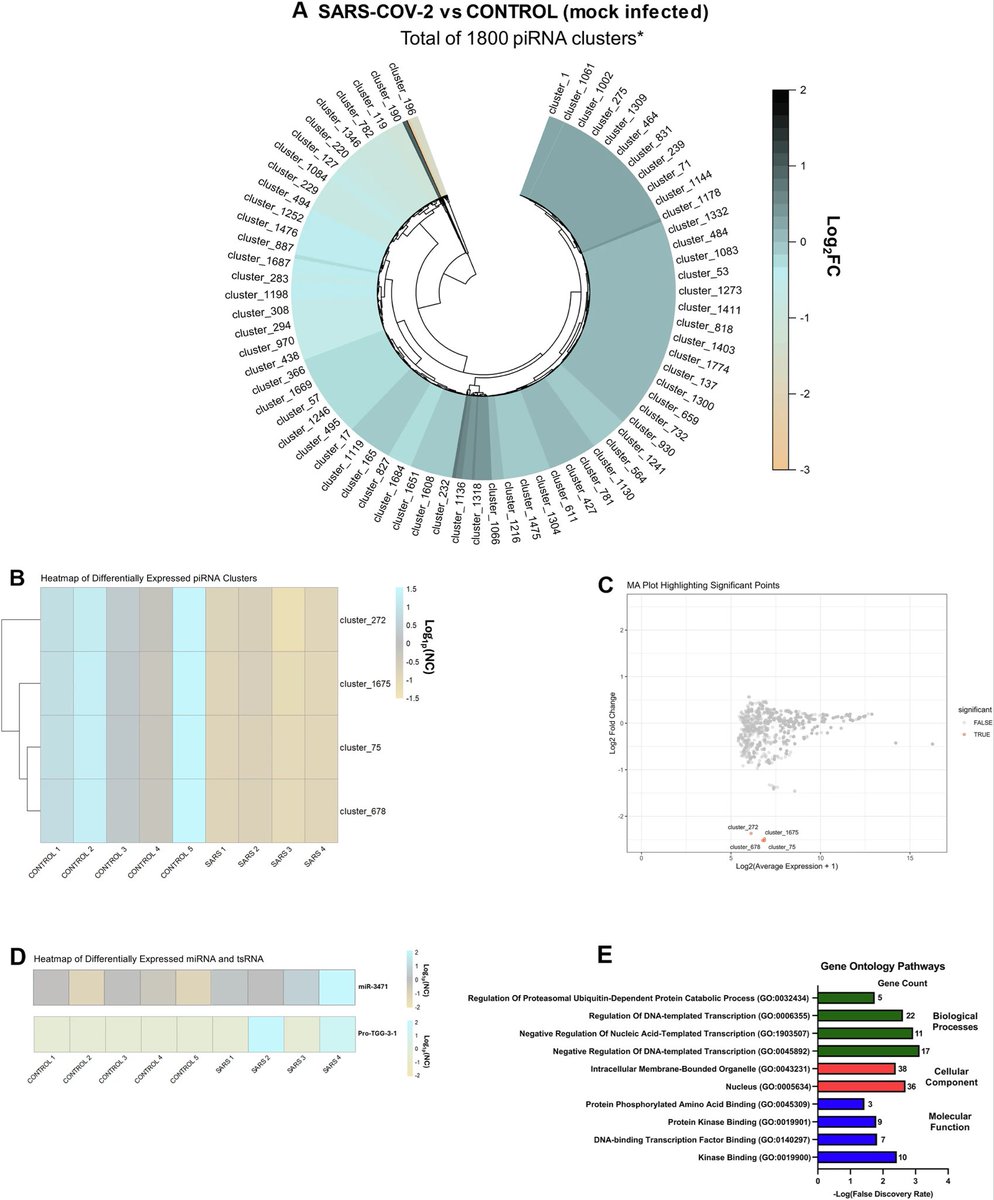
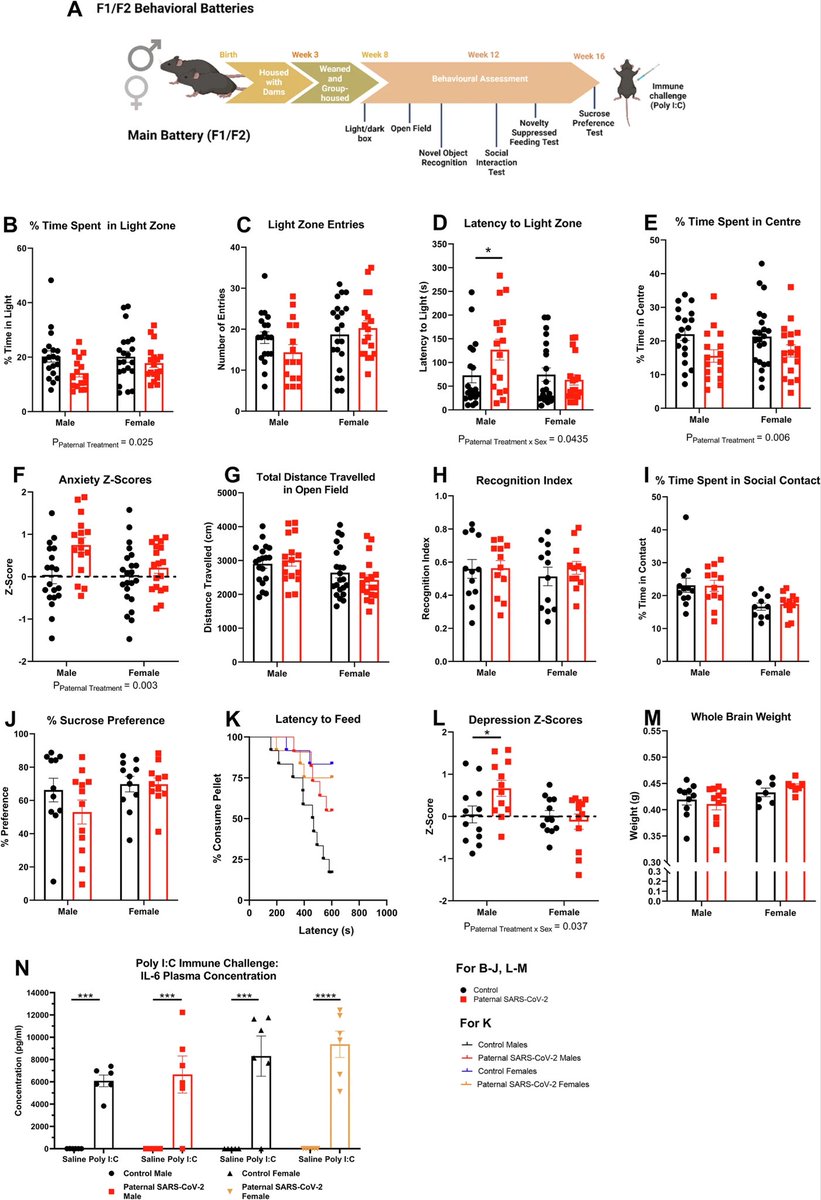
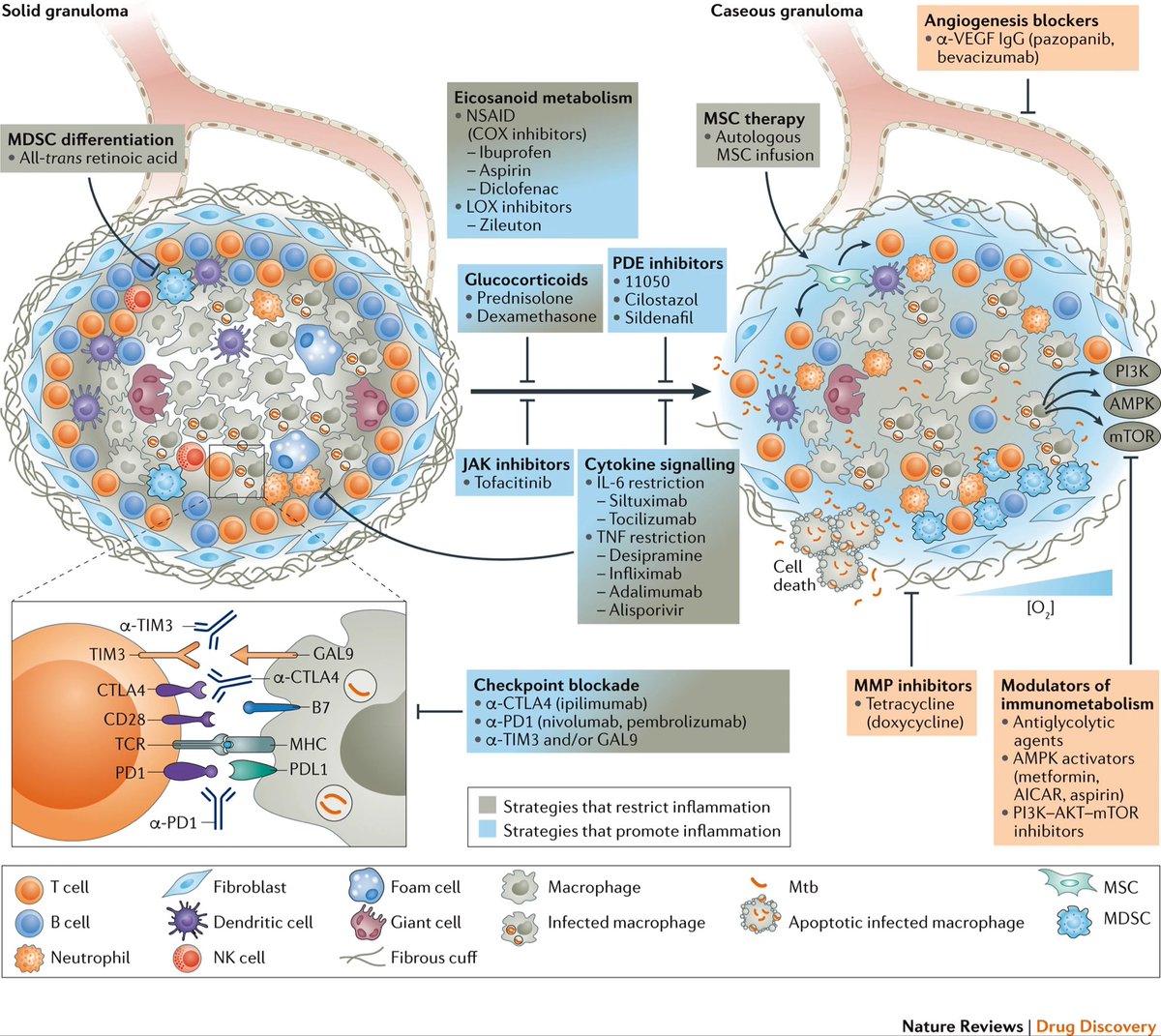
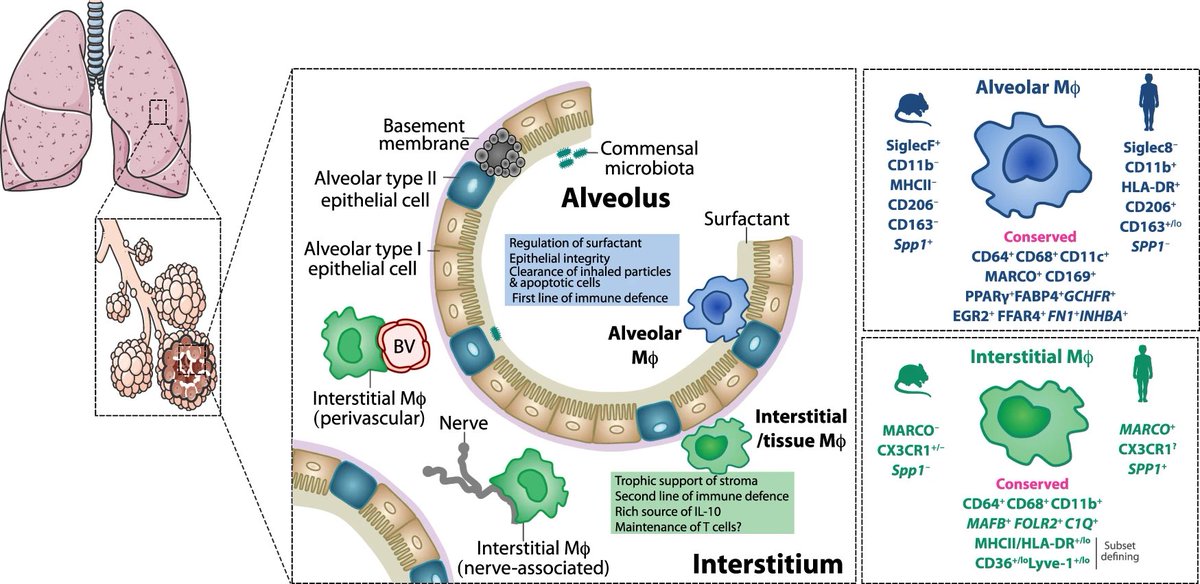
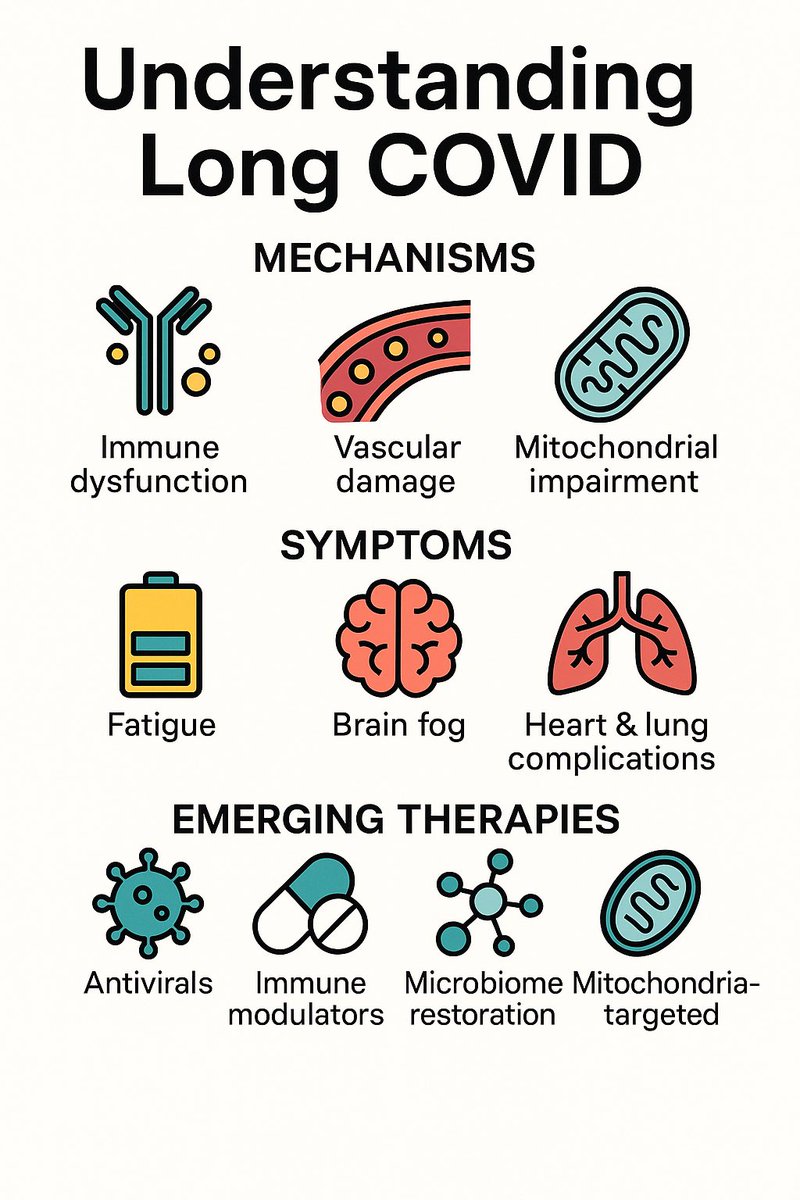
Understanding Long COVID
➡️ Long COVID isn’t one disease — it’s a complex web of immune, vascular, and metabolic dysfunctions.
From fatigue & brain fog to heart & lung complications, it stems from viral persistence, autoimmunity, and mitochondrial damage. 1/
➡️ Long COVID isn’t one disease — it’s a complex web of immune, vascular, and metabolic dysfunctions.
From fatigue & brain fog to heart & lung complications, it stems from viral persistence, autoimmunity, and mitochondrial damage. 1/
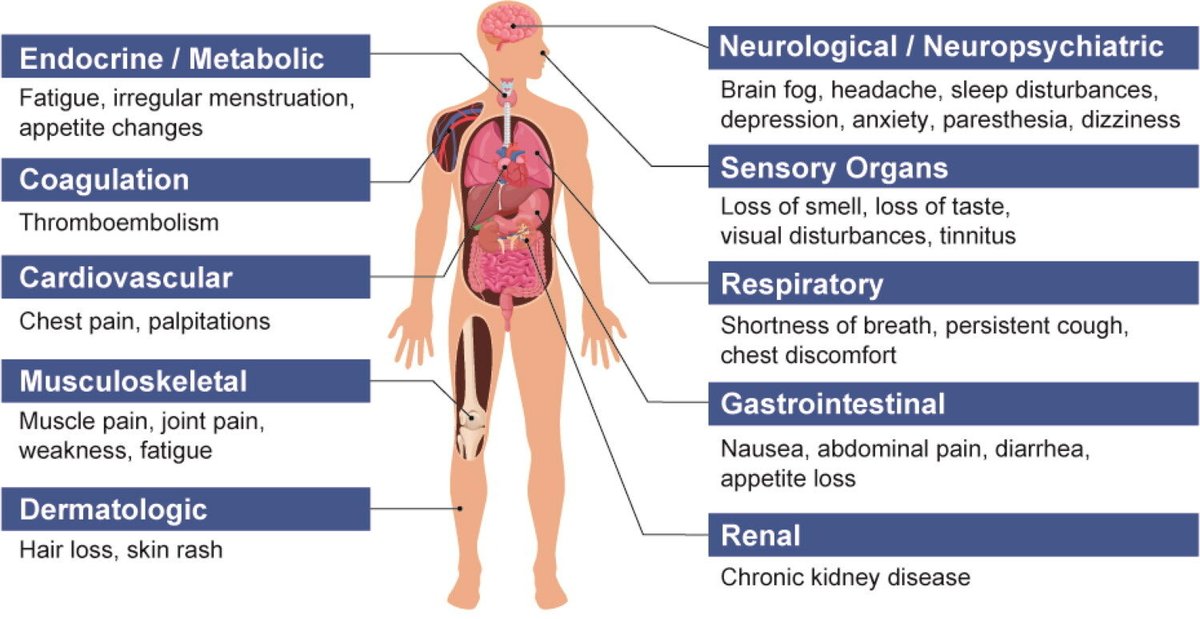
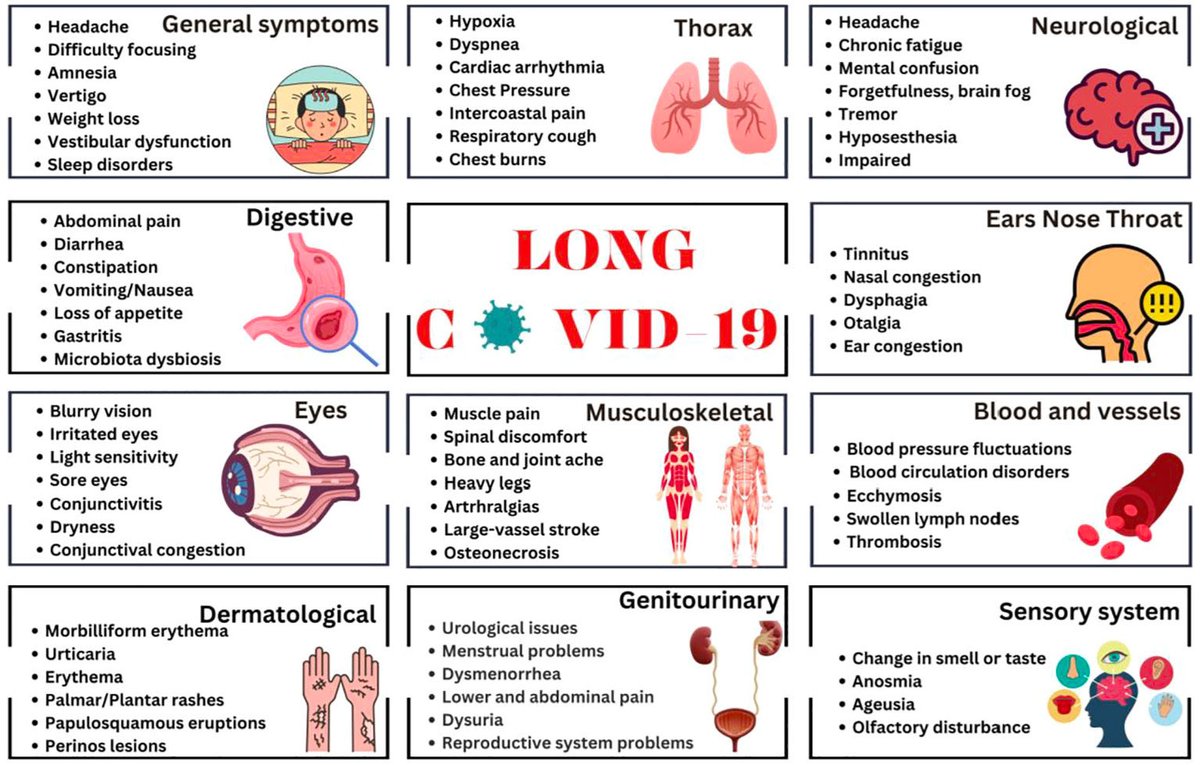
Proposed mechanisms:
1️⃣ Persistent viral reservoirs or antigen remnants
2️⃣ Reactivation of latent viruses (e.g., EBV)
3️⃣ Immune dysregulation & autoimmunity
4️⃣ Endothelial injury and microclots
5️⃣ Gut microbiome imbalance
6️⃣ Mitochondrial dysfunction and energy metabolism impairment. 2/
1️⃣ Persistent viral reservoirs or antigen remnants
2️⃣ Reactivation of latent viruses (e.g., EBV)
3️⃣ Immune dysregulation & autoimmunity
4️⃣ Endothelial injury and microclots
5️⃣ Gut microbiome imbalance
6️⃣ Mitochondrial dysfunction and energy metabolism impairment. 2/
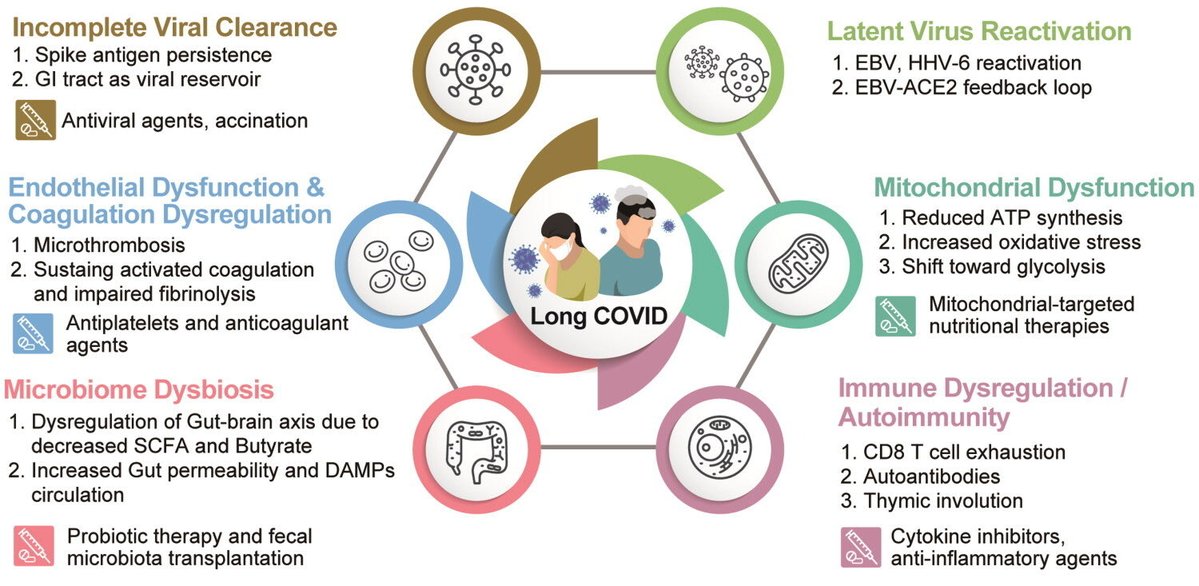
Current management:
- largely symptomatic—rehabilitation, pacing, and supportive therapies.
-Emerging treatments: under study — antiviral drugs, immune-modulating agents, microbiome restoration, and mitochondria-targeted therapies.
-Vaccination: reduces risk and severity of LongCOVID. 3/
- largely symptomatic—rehabilitation, pacing, and supportive therapies.
-Emerging treatments: under study — antiviral drugs, immune-modulating agents, microbiome restoration, and mitochondria-targeted therapies.
-Vaccination: reduces risk and severity of LongCOVID. 3/
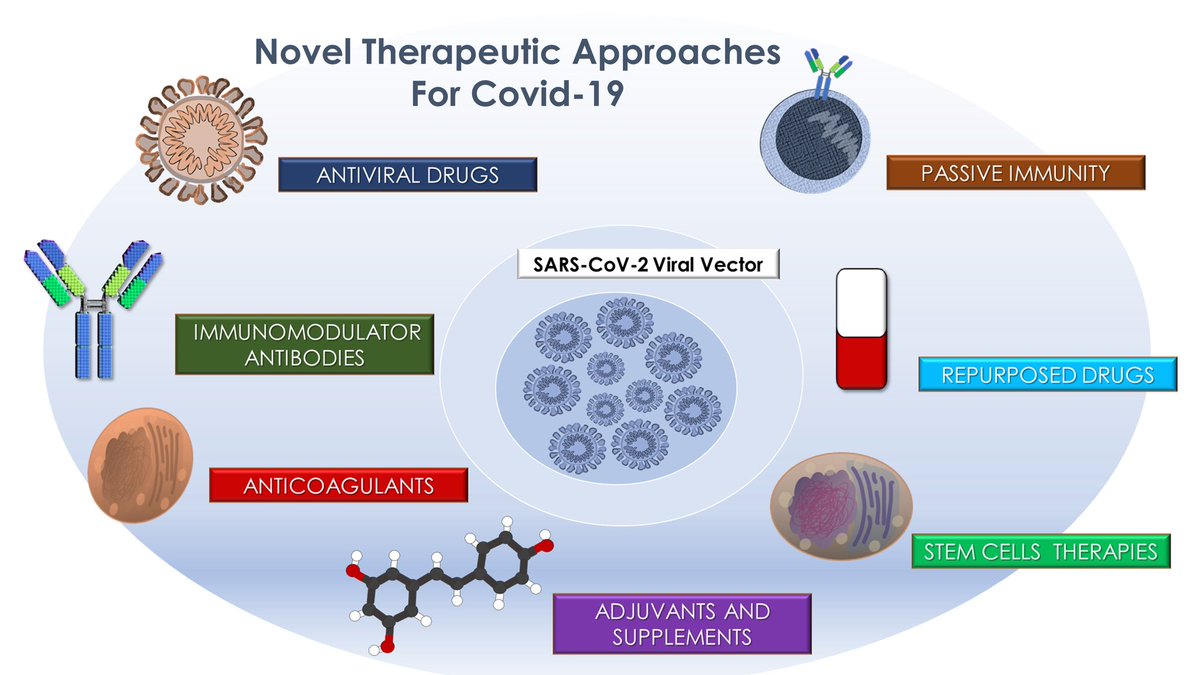
A global health challenge
➡️ #LongCovid affects millions — regardless of how mild the initial infection was.
➡️ Vaccination lowers the risk, but many still suffer lingering inflammation & multi-organ issues.
➡️ We must invest in mechanism-driven, integrative treatments. 4/
➡️ #LongCovid affects millions — regardless of how mild the initial infection was.
➡️ Vaccination lowers the risk, but many still suffer lingering inflammation & multi-organ issues.
➡️ We must invest in mechanism-driven, integrative treatments. 4/
Takeaway
➡️ #LongCOVID is a multi-mechanistic, chronic condition involving immune, vascular, and metabolic pathways.
Future breakthroughs will likely come from integrative approaches targeting viral persistence, immune balance, and cellular energy repair. 5/5
tandfonline.com/doi/full/10.10…
➡️ #LongCOVID is a multi-mechanistic, chronic condition involving immune, vascular, and metabolic pathways.
Future breakthroughs will likely come from integrative approaches targeting viral persistence, immune balance, and cellular energy repair. 5/5
tandfonline.com/doi/full/10.10…
• • •
Missing some Tweet in this thread? You can try to
force a refresh