Understanding the topic of mitochondria is probably the best thing you can do if you want to improve your health.
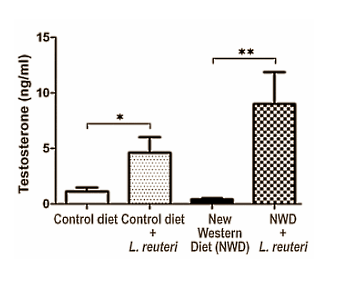
After all, mitochondrial dysfunction is implicated in a host of health conditions ranging from chronic fatigue, low testosterone, depression, bipolar disorders, low testosterone and neurodegenerative diseases all the way to cardiovascular issues, diabetes and even sleep apnea.
Now, what are mitochondria?
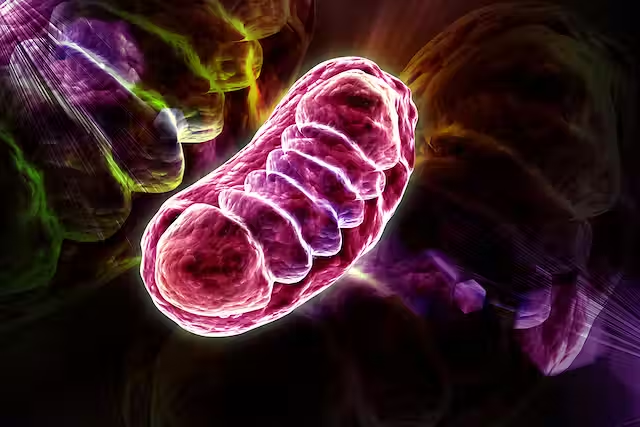
Mitochondria are subcellular organelles that likely originated from ancient α-proteobacteria engulfed by eukaryotic cells.
These organelles produce the vast majority of cellular energy through adenosine triphosphate (ATP), which is needed to power every cell's biochemical reactions.
They also modulate processes like cell signaling, calcium homeostasis and apoptosis.
So it’s really no wonder that mitochondrial dysfunction is implicated in a host of health conditions.
When it comes to the structure of these double-membrane organelles, it’s a good idea to be aware of the following.
We have the:
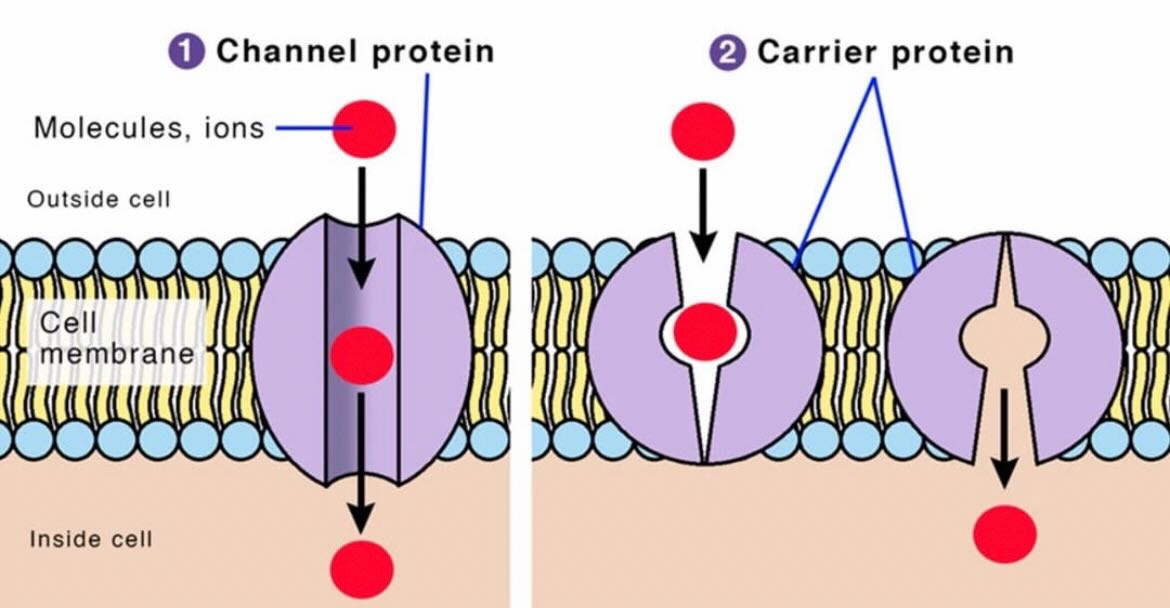
-Outer membrane that is highly permeable due to porins such as voltage-dependent anion channels that allow small molecules and ions to pass freely.
-Inner membrane that is less permeable, with selective transporters, that houses the electron transport chain (ETC) and ATP synthase.
-Intermembrane space that is the region between the membranes.
This one is enriched with protons during ATP synthesis, creating a gradient essential for energy production through chemiosmosis.
-Mitochondrial matrix that is the innermost compartment, containing mitochondrial DNA (mtDNA), 70S ribosomes and enzymes for metabolic pathways like the Krebs cycle.


After all, mitochondrial dysfunction is implicated in a host of health conditions ranging from chronic fatigue, low testosterone, depression, bipolar disorders, low testosterone and neurodegenerative diseases all the way to cardiovascular issues, diabetes and even sleep apnea.
Now, what are mitochondria?
Mitochondria are subcellular organelles that likely originated from ancient α-proteobacteria engulfed by eukaryotic cells.
These organelles produce the vast majority of cellular energy through adenosine triphosphate (ATP), which is needed to power every cell's biochemical reactions.
They also modulate processes like cell signaling, calcium homeostasis and apoptosis.
So it’s really no wonder that mitochondrial dysfunction is implicated in a host of health conditions.
When it comes to the structure of these double-membrane organelles, it’s a good idea to be aware of the following.
We have the:
-Outer membrane that is highly permeable due to porins such as voltage-dependent anion channels that allow small molecules and ions to pass freely.
-Inner membrane that is less permeable, with selective transporters, that houses the electron transport chain (ETC) and ATP synthase.
-Intermembrane space that is the region between the membranes.
This one is enriched with protons during ATP synthesis, creating a gradient essential for energy production through chemiosmosis.
-Mitochondrial matrix that is the innermost compartment, containing mitochondrial DNA (mtDNA), 70S ribosomes and enzymes for metabolic pathways like the Krebs cycle.


Now let’s dive a bit deeper into their main functions.
Let's start with ATP production.
Our cells require, well, energy in order to run properly.
Mitochondria produce ATP through oxidative phosphorylation in the ETC.
How?
In a nutshell, electrons from NADH (complex I) and FADH₂ (complex II) pass through complexes III and IV, pumping protons into the intermembrane space. The resulting proton gradient drives ATP synthase to convert ADP and inorganic phosphate (Pi) into ATP.
If you have no idea what these are, ATP production happens primarily through three stages:
-Glycolysis (happens in the cytoplasm)
-The citric acid cycle (or the Krebs cycle (happens in the mitochondrial matrix))
-Oxidative phosphorylation (happens across the mitochondrial inner membrane)
Glycolysis is anaerobic (no oxygen needed) and takes one glucose molecule breaks it into two 3-carbon pyruvate molecules through a 10-step enzymatic process (glucose gets two phosphates added whcich uses 2 ATP and gives us fructose-1,6-bisphosphate which splits into dihydroxyacetone phosphate and glyceraldehyde-3-phosphate, then the former also converts to G3P, so we get two G3Ps and each one is then oxidized (loses electrons to NAD⁺ and forms 2 NADH (these basically “carry” energy)).
Finally phosphates are transferred to ADP making 4 ATP total (only 2 were used). After some shuffling, you’re left with 2 pyruvate.
Now, we take these 2 pyruvate molecules and each one is converted to acetyl-CoA by pyruvate dehydrogenase. This process releases CO₂ and generates 2 NADH.
For each acetyl-CoA, the following happens: Acetyl-CoA and oxaloacetate get together to form citrate which reshuffles into isocitrate, then isocitrate loses CO₂ and electrons, forming α-ketoglutarate and 1 NADH.
Now that the first oxidation is done, we move to the second one were α-Ketoglutarate drops another CO₂, yielding succinyl-CoA and 1 NADH. Now in this critical step, succinyl-CoA transfers a phosphate to GDP (making GTP, which converts to 1 ATP).
The oxidations don’t stop here and succinate becomes fumarate (1 FADH₂), then malate, then oxaloacetate (1 NADH), completing the loop.
And we can finally talk about oxidative phosphorylation and its two parts, the electron transport chain (ETC) and chemiosmosis. The first one is a series of protein complexes (I-IV) and carriers (ubiquinone, cytochrome c) embedded in the inner mitochondrial membrane.
NADH is used in Complex I and FADH₂ in Complex II.
Ubiquionone also plays a critical role (Complex I → Ubiquinone (Q) → Complex III → Cytochrome c → Complex IV and at Complex IV, electrons combine with O₂ and H⁺ to form deueterium depleted H₂O (oxygen is the final electron acceptor).
In some texts, you will find that metabolic reactions such as converting pyruvate to acetyl-CoA, the citric acid cycle and pyruvic oxidation are mentioned on top of ATP production so here's some further analysis in these for the ones interested.
Pyruvic acid is the simplest of the alpha-keto acids, with a carboxylic acid and a ketone functional group.
The conjugate base, CH3COCOO−, is an intermediate in several metabolic pathways throughout the cell.
Pyruvic acid can be made from glucose through glycolysis, converted back to carbohydrates (such as glucose) via gluconeogenesis, or to fatty acids through a reaction with acetyl-CoA.
It can also be used to construct the amino acid alanine and can be converted into ethanol or lactic acid via fermentation.
It supplies energy to cells through the citric acid cycle (the Krebs cycle) when oxygen is present (aerobic respiration) and alternatively ferments to produce lactate when oxygen is lacking.
In pyruvic oxidation, we are starting with a molecule of pyruvate which has 3 carbons.
Then, we’ll make a molecule of acetyl by dropping a carbon and the carbon that is lost will be lost as a molecule of CO2.
All of these carbon atoms have high energy electrons in their orbitals.
NAD will take the electron that became available through the above process for us to utilize and since it now has an electron on it, it becomes NADH.
So NADH is the product and NAD the reactant.
Now acetyl’s destination is the mitochondria. In order for this to happen, CoA will bind to acetyl and is going to produce acetyl-CoA which can now be accepted by the membrane of the mitochondria and now we can start the next step which is the citric acid cycle.
So, pyruvic acid supplies energy to cells through the citric acid cycle (Krebs cycle) when oxygen is present (aerobic respiration), and alternatively ferments to produce lactate when oxygen is lacking.
A pyruvate carboxylase deficiency, will cause lactic acid to accumulate in the blood, it can damage the body's organs and particularly the nervous system.
In aerobic conditions, the process converts one molecule of glucose into two molecules of pyruvate (pyruvic acid), generating energy in the form of two net molecules of ATP.
Four molecules of ATP per glucose are actually produced, but two are consumed as part of the preparatory phase.
The initial phosphorylation of glucose is required to increase the reactivity (decrease its stability) in order for the molecule to be cleaved into two pyruvate molecules by the enzyme aldolase.
During the pay-off phase of glycolysis, four phosphate groups are transferred to ADP by substrate-level phosphorylation to make four ATP, and two NADH are produced when the pyruvate is oxidized.
The overall reaction can be expressed this way:
Glucose + 2 NAD+ + 2 Pi + 2 ADP → 2 pyruvate + 2 H+ + 2 NADH + 2 ATP + 2 H+ + 2 H2O + energy
Starting with glucose, 1 ATP is used to donate a phosphate to glucose to produce glucose 6-phosphate.
Glycogen can be converted into glucose 6-phosphate as well with the help of glycogen phosphorylase.
During energy metabolism, glucose 6-phosphate becomes fructose 6-phosphate. An additional ATP is used to phosphorylate fructose 6-phosphate into fructose 1,6-bisphosphate by the help of phosphofructokinase.
Fructose 1,6-bisphosphate then splits into two phosphorylated molecules with three carbon chains which later degrades into pyruvate.

Let's start with ATP production.
Our cells require, well, energy in order to run properly.
Mitochondria produce ATP through oxidative phosphorylation in the ETC.
How?
In a nutshell, electrons from NADH (complex I) and FADH₂ (complex II) pass through complexes III and IV, pumping protons into the intermembrane space. The resulting proton gradient drives ATP synthase to convert ADP and inorganic phosphate (Pi) into ATP.
If you have no idea what these are, ATP production happens primarily through three stages:
-Glycolysis (happens in the cytoplasm)
-The citric acid cycle (or the Krebs cycle (happens in the mitochondrial matrix))
-Oxidative phosphorylation (happens across the mitochondrial inner membrane)
Glycolysis is anaerobic (no oxygen needed) and takes one glucose molecule breaks it into two 3-carbon pyruvate molecules through a 10-step enzymatic process (glucose gets two phosphates added whcich uses 2 ATP and gives us fructose-1,6-bisphosphate which splits into dihydroxyacetone phosphate and glyceraldehyde-3-phosphate, then the former also converts to G3P, so we get two G3Ps and each one is then oxidized (loses electrons to NAD⁺ and forms 2 NADH (these basically “carry” energy)).
Finally phosphates are transferred to ADP making 4 ATP total (only 2 were used). After some shuffling, you’re left with 2 pyruvate.
Now, we take these 2 pyruvate molecules and each one is converted to acetyl-CoA by pyruvate dehydrogenase. This process releases CO₂ and generates 2 NADH.
For each acetyl-CoA, the following happens: Acetyl-CoA and oxaloacetate get together to form citrate which reshuffles into isocitrate, then isocitrate loses CO₂ and electrons, forming α-ketoglutarate and 1 NADH.
Now that the first oxidation is done, we move to the second one were α-Ketoglutarate drops another CO₂, yielding succinyl-CoA and 1 NADH. Now in this critical step, succinyl-CoA transfers a phosphate to GDP (making GTP, which converts to 1 ATP).
The oxidations don’t stop here and succinate becomes fumarate (1 FADH₂), then malate, then oxaloacetate (1 NADH), completing the loop.
And we can finally talk about oxidative phosphorylation and its two parts, the electron transport chain (ETC) and chemiosmosis. The first one is a series of protein complexes (I-IV) and carriers (ubiquinone, cytochrome c) embedded in the inner mitochondrial membrane.
NADH is used in Complex I and FADH₂ in Complex II.
Ubiquionone also plays a critical role (Complex I → Ubiquinone (Q) → Complex III → Cytochrome c → Complex IV and at Complex IV, electrons combine with O₂ and H⁺ to form deueterium depleted H₂O (oxygen is the final electron acceptor).
In some texts, you will find that metabolic reactions such as converting pyruvate to acetyl-CoA, the citric acid cycle and pyruvic oxidation are mentioned on top of ATP production so here's some further analysis in these for the ones interested.
Pyruvic acid is the simplest of the alpha-keto acids, with a carboxylic acid and a ketone functional group.
The conjugate base, CH3COCOO−, is an intermediate in several metabolic pathways throughout the cell.
Pyruvic acid can be made from glucose through glycolysis, converted back to carbohydrates (such as glucose) via gluconeogenesis, or to fatty acids through a reaction with acetyl-CoA.
It can also be used to construct the amino acid alanine and can be converted into ethanol or lactic acid via fermentation.
It supplies energy to cells through the citric acid cycle (the Krebs cycle) when oxygen is present (aerobic respiration) and alternatively ferments to produce lactate when oxygen is lacking.
In pyruvic oxidation, we are starting with a molecule of pyruvate which has 3 carbons.
Then, we’ll make a molecule of acetyl by dropping a carbon and the carbon that is lost will be lost as a molecule of CO2.
All of these carbon atoms have high energy electrons in their orbitals.
NAD will take the electron that became available through the above process for us to utilize and since it now has an electron on it, it becomes NADH.
So NADH is the product and NAD the reactant.
Now acetyl’s destination is the mitochondria. In order for this to happen, CoA will bind to acetyl and is going to produce acetyl-CoA which can now be accepted by the membrane of the mitochondria and now we can start the next step which is the citric acid cycle.
So, pyruvic acid supplies energy to cells through the citric acid cycle (Krebs cycle) when oxygen is present (aerobic respiration), and alternatively ferments to produce lactate when oxygen is lacking.
A pyruvate carboxylase deficiency, will cause lactic acid to accumulate in the blood, it can damage the body's organs and particularly the nervous system.
In aerobic conditions, the process converts one molecule of glucose into two molecules of pyruvate (pyruvic acid), generating energy in the form of two net molecules of ATP.
Four molecules of ATP per glucose are actually produced, but two are consumed as part of the preparatory phase.
The initial phosphorylation of glucose is required to increase the reactivity (decrease its stability) in order for the molecule to be cleaved into two pyruvate molecules by the enzyme aldolase.
During the pay-off phase of glycolysis, four phosphate groups are transferred to ADP by substrate-level phosphorylation to make four ATP, and two NADH are produced when the pyruvate is oxidized.
The overall reaction can be expressed this way:
Glucose + 2 NAD+ + 2 Pi + 2 ADP → 2 pyruvate + 2 H+ + 2 NADH + 2 ATP + 2 H+ + 2 H2O + energy
Starting with glucose, 1 ATP is used to donate a phosphate to glucose to produce glucose 6-phosphate.
Glycogen can be converted into glucose 6-phosphate as well with the help of glycogen phosphorylase.
During energy metabolism, glucose 6-phosphate becomes fructose 6-phosphate. An additional ATP is used to phosphorylate fructose 6-phosphate into fructose 1,6-bisphosphate by the help of phosphofructokinase.
Fructose 1,6-bisphosphate then splits into two phosphorylated molecules with three carbon chains which later degrades into pyruvate.


Moving on to the citric acid cycle.
The citric acid cycle (or else known as the tricarboxylic acid cycle or the Krebs’ cycle) is the major energy yielding metabolic pathway in cells, it is an important part of aerobic respiration and takes place in the matrix of the mitochondria (just like the conversion of pyruvate to acetyl CoA).
In order for ATP to be produced through oxidative phosphorylation, electrons are required for ATP to pass down the electron transport chain.
These electrons come from electron carriers such as NADH and FADH, which are produced by the citric acid cycle .
The citric acid cycle harnesses the available chemical energy of acetyl coenzyme A (acetyl CoA) into the reducing power of nicotinamide adenine dinucleotide (NADH).
Here are some of the basic steps of the cycle.
Step 1: The cycle begins with an enzymatic aldol addition reaction of acetyl CoA to oxaloacetate, forming citrate which is isomerized by a dehydration-hydration sequence to yield (2R,3S)-isocitrate.
Step 2: Citrate is converted to isocitrate .
Step 3: Isocitrate is oxidized to alpha-ketoglutarate which results in the release of carbon dioxide. One NADH molecule is then formed.
Step 4: Alpha-ketoglutarate is oxidized to form a 4 carbon molecule in order to bind to coenzyme A, forming succinyl CoA. A second molecule of NADH and CO2 are then being produced.
Step 5: Succinyl CoA is then converted to succinate and one GTP molecule is produced.
Step 6: Succinate is converted into fumarate and a molecule of FADH is produced.
Step 7: Fumarate is converted to malate.
Step 8: Malate is then converted into oxaloacetate.
The third molecule of NADH is also produced.
So, the products of the first turn of the cycle are one ATP, three NADH, one FADH2 and two CO2.
The total number of ATP molecules obtained after complete oxidation of one glucose in glycolysis, citric acid cycle, and oxidative phosphorylation is estimated to be between 30 and 38.
Note: Calcium is also used as a regulator in the citric acid cycle.
Calcium levels in the mitochondrial matrix can reach up to the tens of micromolar levels during cellular activation.
The citric acid cycle (or else known as the tricarboxylic acid cycle or the Krebs’ cycle) is the major energy yielding metabolic pathway in cells, it is an important part of aerobic respiration and takes place in the matrix of the mitochondria (just like the conversion of pyruvate to acetyl CoA).
In order for ATP to be produced through oxidative phosphorylation, electrons are required for ATP to pass down the electron transport chain.
These electrons come from electron carriers such as NADH and FADH, which are produced by the citric acid cycle .
The citric acid cycle harnesses the available chemical energy of acetyl coenzyme A (acetyl CoA) into the reducing power of nicotinamide adenine dinucleotide (NADH).
Here are some of the basic steps of the cycle.
Step 1: The cycle begins with an enzymatic aldol addition reaction of acetyl CoA to oxaloacetate, forming citrate which is isomerized by a dehydration-hydration sequence to yield (2R,3S)-isocitrate.
Step 2: Citrate is converted to isocitrate .
Step 3: Isocitrate is oxidized to alpha-ketoglutarate which results in the release of carbon dioxide. One NADH molecule is then formed.
Step 4: Alpha-ketoglutarate is oxidized to form a 4 carbon molecule in order to bind to coenzyme A, forming succinyl CoA. A second molecule of NADH and CO2 are then being produced.
Step 5: Succinyl CoA is then converted to succinate and one GTP molecule is produced.
Step 6: Succinate is converted into fumarate and a molecule of FADH is produced.
Step 7: Fumarate is converted to malate.
Step 8: Malate is then converted into oxaloacetate.
The third molecule of NADH is also produced.
So, the products of the first turn of the cycle are one ATP, three NADH, one FADH2 and two CO2.
The total number of ATP molecules obtained after complete oxidation of one glucose in glycolysis, citric acid cycle, and oxidative phosphorylation is estimated to be between 30 and 38.
Note: Calcium is also used as a regulator in the citric acid cycle.
Calcium levels in the mitochondrial matrix can reach up to the tens of micromolar levels during cellular activation.

Other key functions of mitochondria include protein transport, apoptosis, calcium homeostasis and the production of ROS.
For example, mitochondria release cytochrome c from the intermembrane space to activate caspases, initiating the intrinsic apoptosis pathway.
This process, regulated by Bcl-2 family proteins, eliminates damaged or unneeded cells.
Them the ETC generates ROS such as superoxide or hydrogen peroxide as byproducts when electrons leak and react with oxygen.
While in moderation, ROS act as signaling molecules, in excess, they cause damage.
For example, mitochondria release cytochrome c from the intermembrane space to activate caspases, initiating the intrinsic apoptosis pathway.
This process, regulated by Bcl-2 family proteins, eliminates damaged or unneeded cells.
Them the ETC generates ROS such as superoxide or hydrogen peroxide as byproducts when electrons leak and react with oxygen.
While in moderation, ROS act as signaling molecules, in excess, they cause damage.

Now let's talk about certain factors that impair mitochondrial function and what we can do about them.
Number 1: Excessive oxidative stress.
Oxidative stress occurs when there is an imbalance between free radical production and their detoxification.
When ROS production is uncontrolled due to low antioxidants for example, it damages mtDNA, proteins and lipids.
Mitochondria are normally protected from oxidative damage by a network of antioxidant systems which consist of superoxide dismutase, catalase, glutathione peroxidase and glutathione reductase together with a number of low molecular weight antioxidants such as α-tocopherol and ubiquinol.
These molecules are particularly effective in scavenging lipid peroxyl radicals and preventing free radical chain reactions of lipid peroxidation
Free radical development is unavoidable, but we have adapted to them by setting up and maintaining defense mechanisms that reduce their impact.
The body’s two major defense systems are free radical detoxifying enzymes which are responsible for protecting the insides of cells from free radical damage and antioxidants which act both inside and outside of cells and block free radicals from stealing electrons by donating one to them.
The three major enzyme systems and the chemical reactions they catalyze are:
1. Superoxide dismutases (SOD).
They are essential for free radical detoxification and these enzymes either have copper (crucial), manganese or zinc as a cofactor.
2. Catalase.
These enzymes convert hydrogen peroxide to water and oxygen and finish the detoxification process that SOD starts.
This enzyme contains iron as a cofactor but since iron can be tricky, don’t simply start upping the high iron foods in order to boost catalase enzymes.
3. Glutathione peroxidases.
These selenium dependent enzymes also convert hydrogen peroxide to water and oxygen.
Antioxidants are broadly classified as either hydrophilic (water soluble) or hydrophobic (lipid soluble), and this classification determines where they act in the body.
Hydrophilic antioxidants act in the cytosol of cells or in extracellular fluids such as blood, hydrophobic antioxidants are largely responsible for protecting cell membranes from free radical damage.
The body can synthesize some antioxidants, but others must be obtained from the diet.
There are two antioxidants that the body synthesizes.
1) Glutathione, which contains a sulfur group that can donate an electron to a free radical, thereby stabilizing it.
2) Uric acid which is too high of a concentration can cause gout.
There are many different antioxidants in food as well.
Antioxidant vitamins (Vitamin E, Vitamin C) donate their electrons to free radicals to stabilize them.
Antioxidant phytochemicals (beta-carotene and other carotenoids) may inhibit the oxidation of lipids or donate electrons.
Antioxidant minerals act as cofactors within complex antioxidant enzyme systems (superoxide dismutases, catalase, glutathione peroxidases described earlier) to convert free radicals to less damaging substances that can be excreted.
Regarding dietary antioxidants, vitamin E protects cellular membranes overall and prevents glutathione depletion.
When we talk about vitamin E, we’re actually referring to 8 chemically similar substances, of which alpha-tocopherol appears to be the most potent antioxidants.
Because vitamin E is fat-soluble, its antioxidant capacity is especially important to lipids, including those in cell membranes and lipoproteins.
For example, free radicals can oxidize LDL cholesterol (stealing an electron from it), and it is this damaged LDL that lodges in blood vessels and forms the fatty plaques characteristic of atherosclerosis, increasing the risk of heart attack, stroke and other complications of cardiovascular disease.
After alpha-tocopherol interacts with a free radical it is no longer capable of acting as an antioxidant unless it is enzymatically regenerated.
Vitamin E, mainly as alpha-tocopherol, plays a role in the immune system, regulation of gene expression, and cell signaling.
Regarding vitamin C, it protects DNA, RNA, proteins and lipids and aids in regenerating vitamin E allowing it to be recycled and used again (after vitamin E donates an electron to neutralize a free radical, it can be regenerated by an electron from vitamin C).
Since it is water-soluble, it acts both inside and outside cells to protect molecules in aqueous environments.
When it comes to selenium, it is an essential trace mineral that is part of the structure of at least 25 proteins in the body with functions in thyroid hormone metabolism, DNA synthesis, reproduction and protecting the immune system.
As part of antioxidant enzymes, selenium helps to regenerate other antioxidants, including vitamin C. These enzymes also protect lipids from free radicals, and, in doing so, spare vitamin E.
Number 1: Excessive oxidative stress.
Oxidative stress occurs when there is an imbalance between free radical production and their detoxification.
When ROS production is uncontrolled due to low antioxidants for example, it damages mtDNA, proteins and lipids.
Mitochondria are normally protected from oxidative damage by a network of antioxidant systems which consist of superoxide dismutase, catalase, glutathione peroxidase and glutathione reductase together with a number of low molecular weight antioxidants such as α-tocopherol and ubiquinol.
These molecules are particularly effective in scavenging lipid peroxyl radicals and preventing free radical chain reactions of lipid peroxidation
Free radical development is unavoidable, but we have adapted to them by setting up and maintaining defense mechanisms that reduce their impact.
The body’s two major defense systems are free radical detoxifying enzymes which are responsible for protecting the insides of cells from free radical damage and antioxidants which act both inside and outside of cells and block free radicals from stealing electrons by donating one to them.
The three major enzyme systems and the chemical reactions they catalyze are:
1. Superoxide dismutases (SOD).
They are essential for free radical detoxification and these enzymes either have copper (crucial), manganese or zinc as a cofactor.
2. Catalase.
These enzymes convert hydrogen peroxide to water and oxygen and finish the detoxification process that SOD starts.
This enzyme contains iron as a cofactor but since iron can be tricky, don’t simply start upping the high iron foods in order to boost catalase enzymes.
3. Glutathione peroxidases.
These selenium dependent enzymes also convert hydrogen peroxide to water and oxygen.
Antioxidants are broadly classified as either hydrophilic (water soluble) or hydrophobic (lipid soluble), and this classification determines where they act in the body.
Hydrophilic antioxidants act in the cytosol of cells or in extracellular fluids such as blood, hydrophobic antioxidants are largely responsible for protecting cell membranes from free radical damage.
The body can synthesize some antioxidants, but others must be obtained from the diet.
There are two antioxidants that the body synthesizes.
1) Glutathione, which contains a sulfur group that can donate an electron to a free radical, thereby stabilizing it.
2) Uric acid which is too high of a concentration can cause gout.
There are many different antioxidants in food as well.
Antioxidant vitamins (Vitamin E, Vitamin C) donate their electrons to free radicals to stabilize them.
Antioxidant phytochemicals (beta-carotene and other carotenoids) may inhibit the oxidation of lipids or donate electrons.
Antioxidant minerals act as cofactors within complex antioxidant enzyme systems (superoxide dismutases, catalase, glutathione peroxidases described earlier) to convert free radicals to less damaging substances that can be excreted.
Regarding dietary antioxidants, vitamin E protects cellular membranes overall and prevents glutathione depletion.
When we talk about vitamin E, we’re actually referring to 8 chemically similar substances, of which alpha-tocopherol appears to be the most potent antioxidants.
Because vitamin E is fat-soluble, its antioxidant capacity is especially important to lipids, including those in cell membranes and lipoproteins.
For example, free radicals can oxidize LDL cholesterol (stealing an electron from it), and it is this damaged LDL that lodges in blood vessels and forms the fatty plaques characteristic of atherosclerosis, increasing the risk of heart attack, stroke and other complications of cardiovascular disease.
After alpha-tocopherol interacts with a free radical it is no longer capable of acting as an antioxidant unless it is enzymatically regenerated.
Vitamin E, mainly as alpha-tocopherol, plays a role in the immune system, regulation of gene expression, and cell signaling.
Regarding vitamin C, it protects DNA, RNA, proteins and lipids and aids in regenerating vitamin E allowing it to be recycled and used again (after vitamin E donates an electron to neutralize a free radical, it can be regenerated by an electron from vitamin C).
Since it is water-soluble, it acts both inside and outside cells to protect molecules in aqueous environments.
When it comes to selenium, it is an essential trace mineral that is part of the structure of at least 25 proteins in the body with functions in thyroid hormone metabolism, DNA synthesis, reproduction and protecting the immune system.
As part of antioxidant enzymes, selenium helps to regenerate other antioxidants, including vitamin C. These enzymes also protect lipids from free radicals, and, in doing so, spare vitamin E.

Number 2: Sleep Disruption and circadian misalignment.
In this one we have an unhealthy light environment (plenty of artificial blue light with too little IR, NIR) that's been clearly shown to harm mitochondria.
The combination of: Poor light environment, poor sleep and circadian misalignment 100% impairs mitochondrial repair.
In this one we have an unhealthy light environment (plenty of artificial blue light with too little IR, NIR) that's been clearly shown to harm mitochondria.
The combination of: Poor light environment, poor sleep and circadian misalignment 100% impairs mitochondrial repair.

Number 3: Poor nutritional habits.
This one includes overconsuming foods, the consumption of highly processed foods, a diet with a high 06 and a low 03 intake, and being deficient in B vitamins, magnesium, copper, zinc, iron and dietary antioxidants.
B vitamins for example are required for pyruvate dehydrogenase , α-ketoglutarate dehydrogenase, FAD, NAD and acetyl-CoA formation.
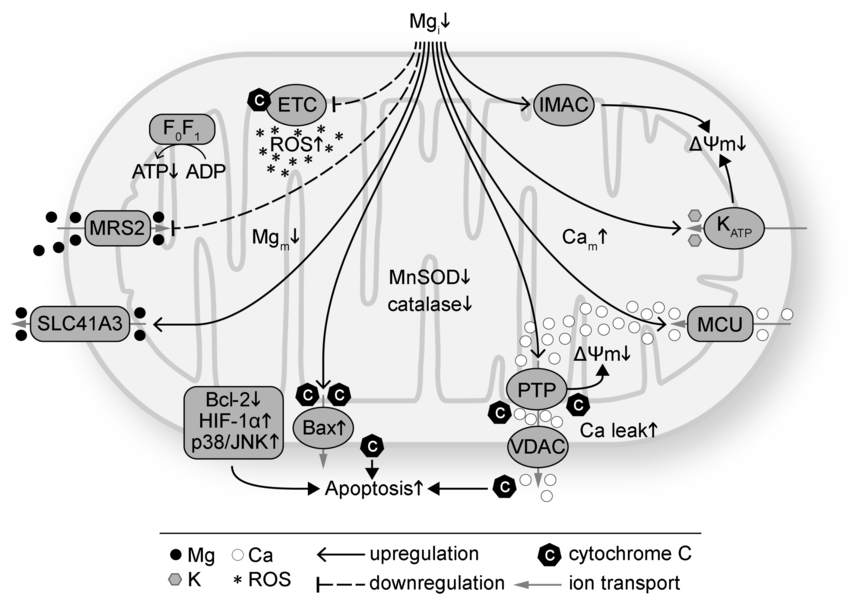
Magnesium is required for ATPase activity, copper is a cofactor for cytochrome c oxidase etc
But i would truly want to hammer home the point of over-eating in general.
In order to understand how important this is, mitophagy is a type of autophagy that is needed to break down dysfunctional or damaged mitochondria.
Guess what upregulates it?
Intermittent fasting (IF) and caloric restriction (CR) that activate pathways like AMP-activated protein kinase (AMPK) and sirtuins such as SIRT1 which upregulate autophagy and mitophagy.
This one includes overconsuming foods, the consumption of highly processed foods, a diet with a high 06 and a low 03 intake, and being deficient in B vitamins, magnesium, copper, zinc, iron and dietary antioxidants.
B vitamins for example are required for pyruvate dehydrogenase , α-ketoglutarate dehydrogenase, FAD, NAD and acetyl-CoA formation.
Magnesium is required for ATPase activity, copper is a cofactor for cytochrome c oxidase etc
But i would truly want to hammer home the point of over-eating in general.
In order to understand how important this is, mitophagy is a type of autophagy that is needed to break down dysfunctional or damaged mitochondria.
Guess what upregulates it?
Intermittent fasting (IF) and caloric restriction (CR) that activate pathways like AMP-activated protein kinase (AMPK) and sirtuins such as SIRT1 which upregulate autophagy and mitophagy.

Number 4: Sedentary lifestyle.
Lack of physical activity reduces mitochondrial biogenesis (creation of new mitochondria).
This is why HIIT for example is shown to increase mitochondria in skeletal muscle.
Overall, HIIT is shown to stimulate mitophagy via PGC-1α and AMPK activation.
Lack of physical activity reduces mitochondrial biogenesis (creation of new mitochondria).
This is why HIIT for example is shown to increase mitochondria in skeletal muscle.
Overall, HIIT is shown to stimulate mitophagy via PGC-1α and AMPK activation.

Number 5: The use of medications such as statins, SSRIs and metformin.
These cause CoQ10 depletion by inhibiting the mevalonate pathway, decrease mtDNA and inhibit the ETC.
If you choose to use them, consider adding ubiquinol, get sun (vitamin D is shown) to prevent the depletion of mtDNA and prioritize B vitamin rich foods.
Then metformin for example inhibits complex I.
These cause CoQ10 depletion by inhibiting the mevalonate pathway, decrease mtDNA and inhibit the ETC.
If you choose to use them, consider adding ubiquinol, get sun (vitamin D is shown) to prevent the depletion of mtDNA and prioritize B vitamin rich foods.
Then metformin for example inhibits complex I.

Number 6: Toxin accumulation.
Mitochondria are the first target sites for a variety of heavy metals for example.
For example, chronic mercury exposure results in its accumulation in mitochondria, which causes ultrastructural mitochondrial alterations that depolarize the mitochondrial membrane and subsequently reduce ATP production and Ca2+ buffering capacity.
Mitochondria are the first target sites for a variety of heavy metals for example.
For example, chronic mercury exposure results in its accumulation in mitochondria, which causes ultrastructural mitochondrial alterations that depolarize the mitochondrial membrane and subsequently reduce ATP production and Ca2+ buffering capacity.

Now 5 more relatively safe tools/ lifestyle interventions to further consider include (besides the things that address what was just mentioned (nutrition, training, sleep etc)).
Number 1: Increasing NAD+
NAD+ for example acts as an electron acceptor in the Krebs cycle and OXPHOS, is consumed by PARPs (enzymes that detect DNA damage) consume it, CD38 and CD157 that modulate immune responses use it, it is required for 3β-HSD to convert pregnenolone to progesterone, SIRT1 and so on.
How the body synthesizes NAD+ 101.
Number 1: De Novo pathway.
This pathway starts with tryptophan, that is converted into quinolinic acid through a series of enzymatic steps involving the kynurenine pathway.
Quinolinic acid is then transformed into nicotinic acid mononucleotide (NaMN) by the enzyme quinolinate
phosphoribosyltransferase (QPRT).
NaMN is further converted to NAD+ via nicotinamide mononucleotide adenylyltransferase (NMNAT).
Note: This pathway is less efficient, contributing only to 10-15% of NAD+ in humans, as it requires significant energy and is tightly regulated.
It’s active primarily in the liver and kidneys, where tryptophan metabolism is robust.
Number 2: Preiss-Handler pathway.
This pathway uses nicotinic acid (NA), a form of vitamin B3 that is converted to NaMN by nicotinate phosphoribosyltransferase (NAPRT).
NaMN is then transformed into NAD+ by NMNAT and NAD+ synthase, requiring magnesium and ATP as cofactors.
This pathway is highly efficient in tissues with high NAPRT expression such as the liver, kidneys and intestines.
It’s a key route for dietary niacin to boost NAD+ levels, but it requires adequate magnesium and ATP as cofactors.
Number 3: Salvage pathway.
The salvage pathway recycles nicotinamide (NAM) and uses precursors like nicotinamide mononucleotide (NMN) and nicotinamide riboside (NR).
Nicotinamide (NAM) is converted to NMN by nicotinamide phosphoribosyltransferase (NAMPT), the rate-limiting enzyme.
NMN is then transformed into NAD+ by NMNAT.
NR is converted to NMN by nicotinamide riboside kinases (NRK1/2), then to NAD+ by NMNAT.
NMN is directly converted to NAD+ by NMNAT, making it the closest precursor to NAD+.
The salvage pathway is the primary route for maintaining NAD+ levels in most tissues, as it recycles NAM produced from NAD+ breakdown by PARPs or sirtuins).
So, if NAD+ is so important, how do we keep NAD+ levels high?
First, before you even think about supplements, focus on lifestyle and nutritional changes that help maximize your body’s ability to synthesize and recycle NAD+.
These include:
-Dietary niacin (a precursor for NAD+ synthesis through the Preiss-Handler pathway)
-Exercise (upregulates NAMPT (an enzyme in the NAD+ salvage pathway))
-B2 (crucial for FAD)
-B9 (supports methylation, which is linked to NAD+ recycling via the methionine cycle)
-B12 (supports methylation, which is linked to NAD+ recycling via the methionine cycle)
-Having a healthy circadian rhythm (NAD+ levels oscillate with the circadian clock, regulated by CLOCK/BMAL1 and SIRT1 interaction)
-Malic acid or succinic acid (an intermediate of the Krebs cycle shown to greatly increase NAD+ and strongly increase the NAD/NADH ratio).
-TMG/glycine (methylation support)
-Sunlight / Grounding
-Vitamin K
-Magnesium (cofactor for NMNAT)
-Avoiding too many acellular carbs/sources of deuterium such as vegetable oils (oxidative stress depletes NAD+ by activating PARPs and CD38)
-Zinc (supports PARP)
-Quitting sources of oxidative stress such as smoking (oxidative stress depletes NAD+ by activating PARPs and CD38))
-Selenium (reduces NAD+ consumption by supporting glutathione peroxidase)
-The occasional sauna (it activates heat shock proteins and may upregulate SIRT1)
-The occasional cold exposure (activates AMPK and SIRT1)
-Some occasional fasting / if your condition allows it (upregulate AMPK and SIRT1,)
-Sprinkling keto during the winter months / if your condition allow it
So basically, when it comes to NAD+, we must first and foremost work on what we synthesize and recycle.
Then, certain polyphenols such as quercetin or apigenin can activate sirtuins or inhibit CD38, thus preserving NAD+.
CoQ10, creatine, blackseed oil and methylene blue can also help.
Now let's see who might benefit from supplementation:
-People with neurological disorders
-Smokers
-People who battle a virus such as Covid-19 or Lyme
-People struggling with chronic fatigue
-People with genetic variants such as rs61330082 and rs1130169 C/C.
How to safely do it:
-Start with just 120-150 mg of NMN and pair it with TMG to reduce potential side effects like homocysteine buildup.
-Start with just 120-150 mg of NMN and pair it with TMG to reduce potential side effects like homocysteine buildup.
-If there's still none, go to 500mg
-Do 2-3 weeks on: 3-4 weeks off cycles
For IVs always contact your doctor since nothing in here should be used as a substitute for medical advice.
Who should NOT use NAD+ related supplements:
-Cancer patients (there are mixed results (especially based on the type of chemotherapy drugs the person might be using), so contact your doctor)
-People with mutations in NAMPT or NMNAT gene
-People with liver diseases such as hepatitis and cirrhosis
-People with unaddressed methylation issues (especially those with unaddressed MTHFR SNPs)
-People using methotrexate or statins (any type)
Note: Why NR or NMN instead of overly expensive NAD+ drips?
1. NAD+ is a large, charged molecule that struggles to pass through intact cell membranes due to its size and polarity.
2. Once ingested, NAD+ is often broken down in the gut or bloodstream into smaller precursors like nicotinamide (NAM), NR, or NMN before cells can use it.
3. Both NR and NMN are smaller molecules that can cross cell membranes more easily via specific transporters (think ENT for NMN or passive diffusion for NR).
Once inside cells, they are efficiently converted into NAD+ through well-established pathways (NR via the NRK pathway, NMN via the NMNAT enzyme).
4. A single dose of NR can increase blood NAD+ levels by up to 2.7-fold and NMN supplementation effectively restores intracellular NAD+ levels, improving mitochondrial function and cognitive outcomes in conditions like Alzheimer’s.
Number 1: Increasing NAD+
NAD+ for example acts as an electron acceptor in the Krebs cycle and OXPHOS, is consumed by PARPs (enzymes that detect DNA damage) consume it, CD38 and CD157 that modulate immune responses use it, it is required for 3β-HSD to convert pregnenolone to progesterone, SIRT1 and so on.
How the body synthesizes NAD+ 101.
Number 1: De Novo pathway.
This pathway starts with tryptophan, that is converted into quinolinic acid through a series of enzymatic steps involving the kynurenine pathway.
Quinolinic acid is then transformed into nicotinic acid mononucleotide (NaMN) by the enzyme quinolinate
phosphoribosyltransferase (QPRT).
NaMN is further converted to NAD+ via nicotinamide mononucleotide adenylyltransferase (NMNAT).
Note: This pathway is less efficient, contributing only to 10-15% of NAD+ in humans, as it requires significant energy and is tightly regulated.
It’s active primarily in the liver and kidneys, where tryptophan metabolism is robust.
Number 2: Preiss-Handler pathway.
This pathway uses nicotinic acid (NA), a form of vitamin B3 that is converted to NaMN by nicotinate phosphoribosyltransferase (NAPRT).
NaMN is then transformed into NAD+ by NMNAT and NAD+ synthase, requiring magnesium and ATP as cofactors.
This pathway is highly efficient in tissues with high NAPRT expression such as the liver, kidneys and intestines.
It’s a key route for dietary niacin to boost NAD+ levels, but it requires adequate magnesium and ATP as cofactors.
Number 3: Salvage pathway.
The salvage pathway recycles nicotinamide (NAM) and uses precursors like nicotinamide mononucleotide (NMN) and nicotinamide riboside (NR).
Nicotinamide (NAM) is converted to NMN by nicotinamide phosphoribosyltransferase (NAMPT), the rate-limiting enzyme.
NMN is then transformed into NAD+ by NMNAT.
NR is converted to NMN by nicotinamide riboside kinases (NRK1/2), then to NAD+ by NMNAT.
NMN is directly converted to NAD+ by NMNAT, making it the closest precursor to NAD+.
The salvage pathway is the primary route for maintaining NAD+ levels in most tissues, as it recycles NAM produced from NAD+ breakdown by PARPs or sirtuins).
So, if NAD+ is so important, how do we keep NAD+ levels high?
First, before you even think about supplements, focus on lifestyle and nutritional changes that help maximize your body’s ability to synthesize and recycle NAD+.
These include:
-Dietary niacin (a precursor for NAD+ synthesis through the Preiss-Handler pathway)
-Exercise (upregulates NAMPT (an enzyme in the NAD+ salvage pathway))
-B2 (crucial for FAD)
-B9 (supports methylation, which is linked to NAD+ recycling via the methionine cycle)
-B12 (supports methylation, which is linked to NAD+ recycling via the methionine cycle)
-Having a healthy circadian rhythm (NAD+ levels oscillate with the circadian clock, regulated by CLOCK/BMAL1 and SIRT1 interaction)
-Malic acid or succinic acid (an intermediate of the Krebs cycle shown to greatly increase NAD+ and strongly increase the NAD/NADH ratio).
-TMG/glycine (methylation support)
-Sunlight / Grounding
-Vitamin K
-Magnesium (cofactor for NMNAT)
-Avoiding too many acellular carbs/sources of deuterium such as vegetable oils (oxidative stress depletes NAD+ by activating PARPs and CD38)
-Zinc (supports PARP)
-Quitting sources of oxidative stress such as smoking (oxidative stress depletes NAD+ by activating PARPs and CD38))
-Selenium (reduces NAD+ consumption by supporting glutathione peroxidase)
-The occasional sauna (it activates heat shock proteins and may upregulate SIRT1)
-The occasional cold exposure (activates AMPK and SIRT1)
-Some occasional fasting / if your condition allows it (upregulate AMPK and SIRT1,)
-Sprinkling keto during the winter months / if your condition allow it
So basically, when it comes to NAD+, we must first and foremost work on what we synthesize and recycle.
Then, certain polyphenols such as quercetin or apigenin can activate sirtuins or inhibit CD38, thus preserving NAD+.
CoQ10, creatine, blackseed oil and methylene blue can also help.
Now let's see who might benefit from supplementation:
-People with neurological disorders
-Smokers
-People who battle a virus such as Covid-19 or Lyme
-People struggling with chronic fatigue
-People with genetic variants such as rs61330082 and rs1130169 C/C.
How to safely do it:
-Start with just 120-150 mg of NMN and pair it with TMG to reduce potential side effects like homocysteine buildup.
-Start with just 120-150 mg of NMN and pair it with TMG to reduce potential side effects like homocysteine buildup.
-If there's still none, go to 500mg
-Do 2-3 weeks on: 3-4 weeks off cycles
For IVs always contact your doctor since nothing in here should be used as a substitute for medical advice.
Who should NOT use NAD+ related supplements:
-Cancer patients (there are mixed results (especially based on the type of chemotherapy drugs the person might be using), so contact your doctor)
-People with mutations in NAMPT or NMNAT gene
-People with liver diseases such as hepatitis and cirrhosis
-People with unaddressed methylation issues (especially those with unaddressed MTHFR SNPs)
-People using methotrexate or statins (any type)
Note: Why NR or NMN instead of overly expensive NAD+ drips?
1. NAD+ is a large, charged molecule that struggles to pass through intact cell membranes due to its size and polarity.
2. Once ingested, NAD+ is often broken down in the gut or bloodstream into smaller precursors like nicotinamide (NAM), NR, or NMN before cells can use it.
3. Both NR and NMN are smaller molecules that can cross cell membranes more easily via specific transporters (think ENT for NMN or passive diffusion for NR).
Once inside cells, they are efficiently converted into NAD+ through well-established pathways (NR via the NRK pathway, NMN via the NMNAT enzyme).
4. A single dose of NR can increase blood NAD+ levels by up to 2.7-fold and NMN supplementation effectively restores intracellular NAD+ levels, improving mitochondrial function and cognitive outcomes in conditions like Alzheimer’s.

Number 5: Acetyl l-carnitine (needs cycling/view it as an intervention and not a staple).
Note: people with hypothyroidism, a history of seizure disorders, people on blood thinners, people struggling with bipolar disorders or are pre-disposed to hypomania and people who are predisposed to TMAO formation should stay away of it (you could manage some of the side effects with alicin for example but the benefits in these cases do not surpass the downsides UNLESS we are talking about the topic of sperm quality since carnitine is just that good with it).
Note: people with hypothyroidism, a history of seizure disorders, people on blood thinners, people struggling with bipolar disorders or are pre-disposed to hypomania and people who are predisposed to TMAO formation should stay away of it (you could manage some of the side effects with alicin for example but the benefits in these cases do not surpass the downsides UNLESS we are talking about the topic of sperm quality since carnitine is just that good with it).

That's it.
These were the basics.
I hope that you found something useful in this thread.
If you did, make sure to leave a like/RT.
These were the basics.
I hope that you found something useful in this thread.
If you did, make sure to leave a like/RT.
https://x.com/Helios_Movement/status/1983591426802778176
• • •
Missing some Tweet in this thread? You can try to
force a refresh