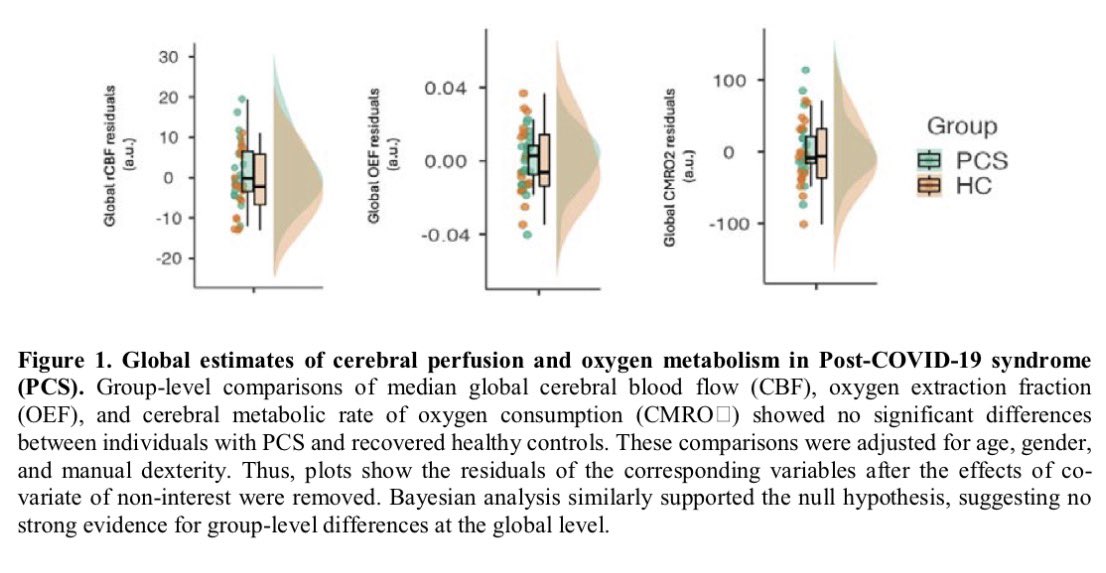
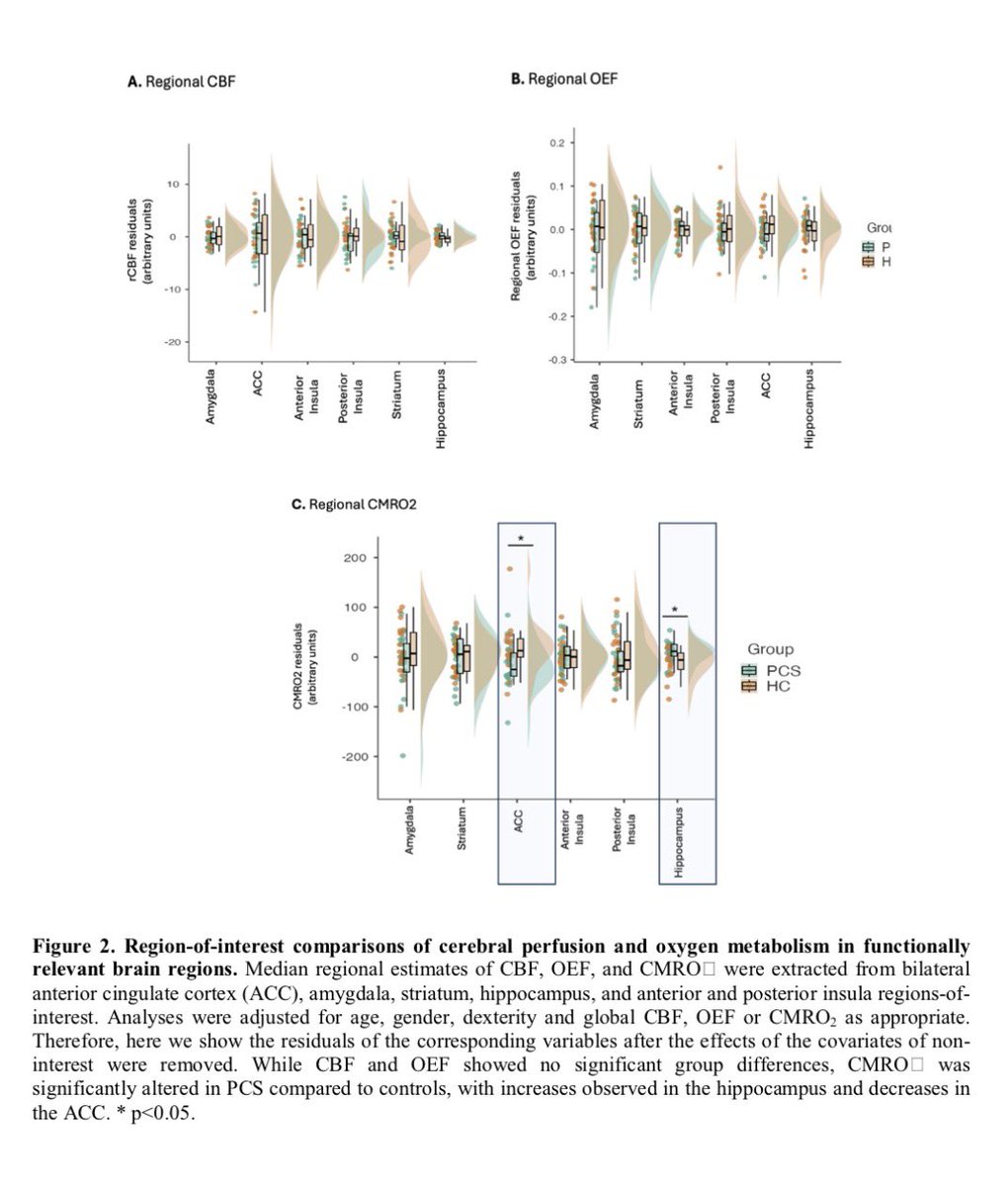
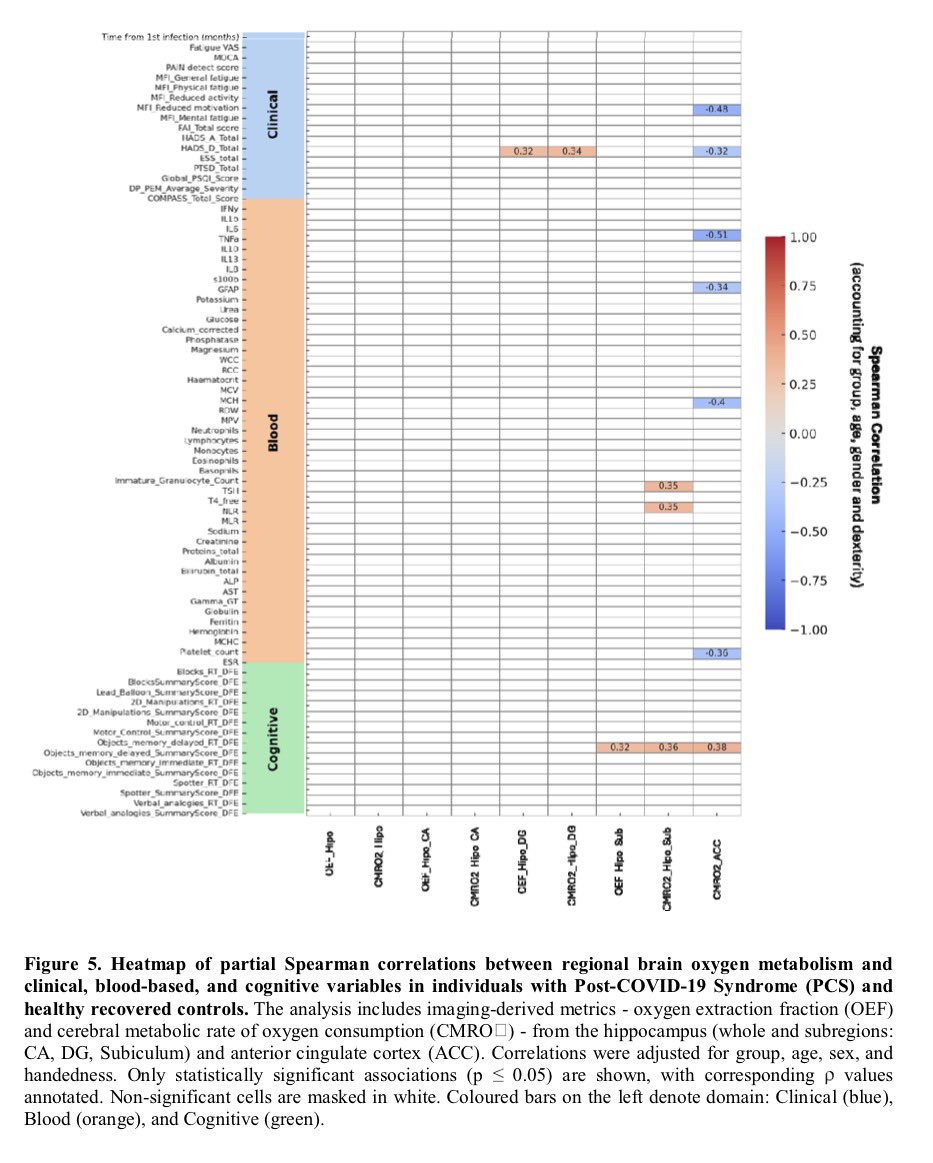
New research in Cell Reports Medicine helps explain why women are more likely to develop #LongCOVID — and often experience more severe, persistent symptoms like fatigue, brain fog, and pain.
The key? Differences in the immune system, gut, and hormones. 1/
The key? Differences in the immune system, gut, and hormones. 1/

Researchers studied 78 people with LongCOVID (mostly mild initial cases) and compared them to 62 who recovered fully.
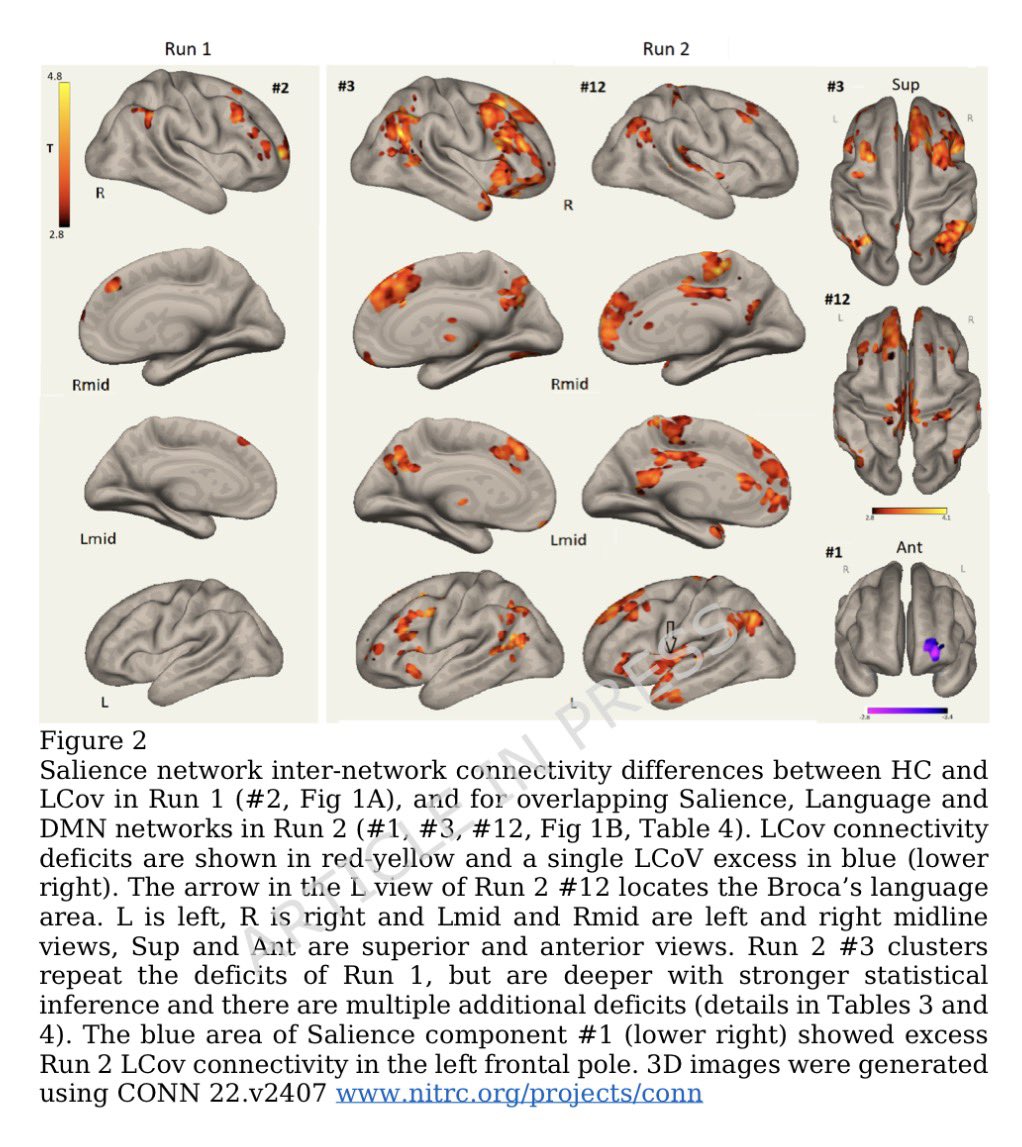
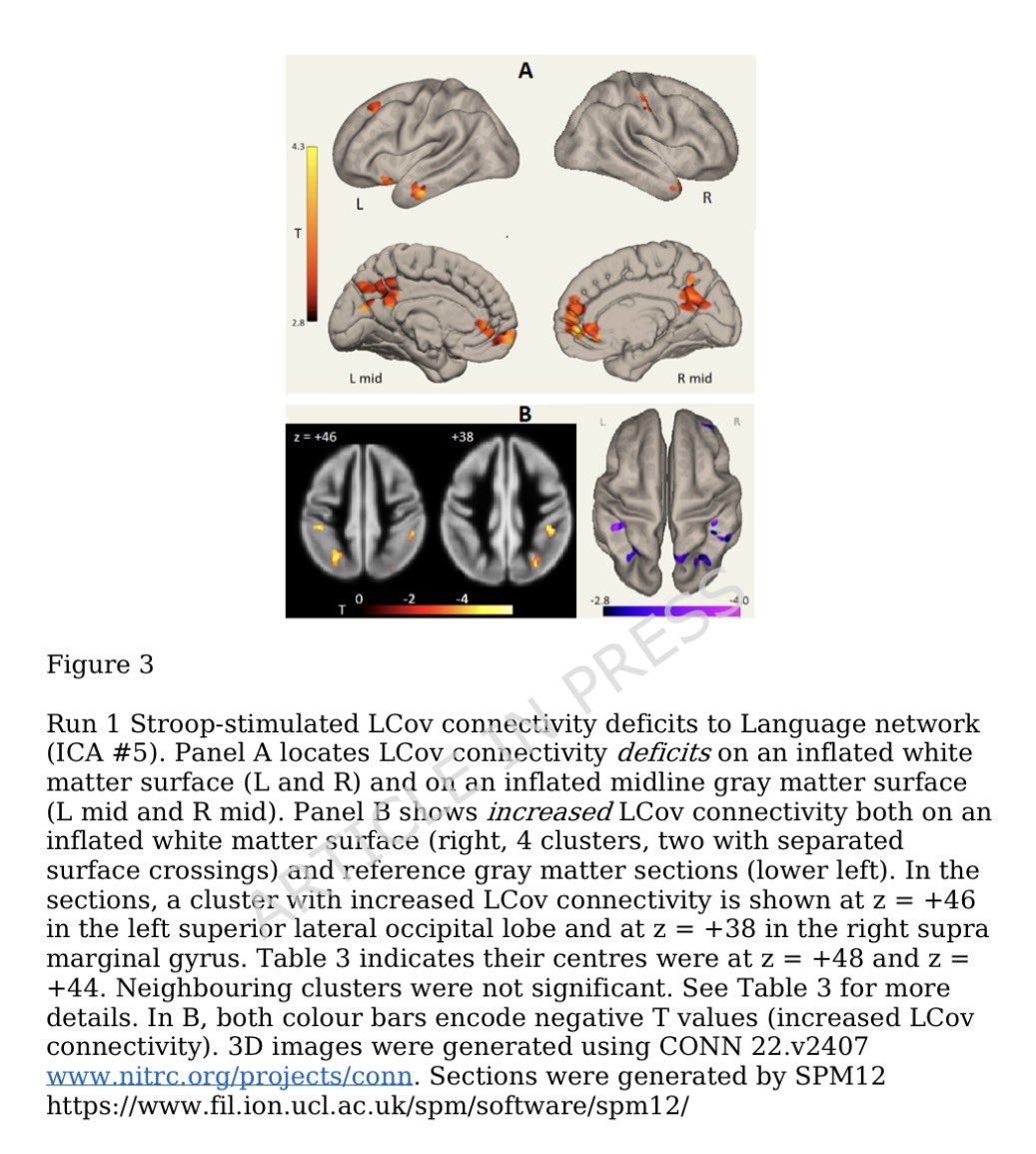
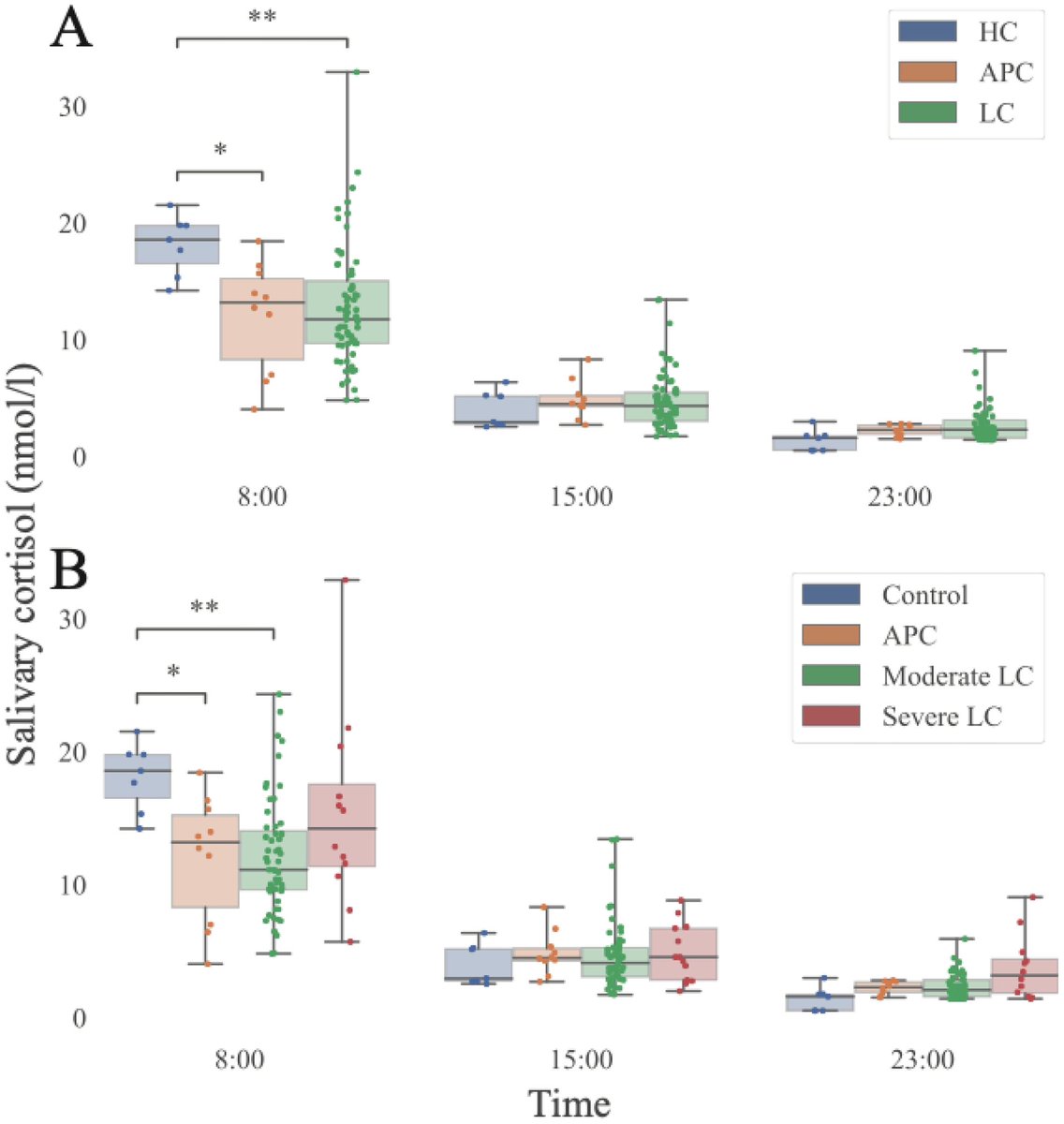
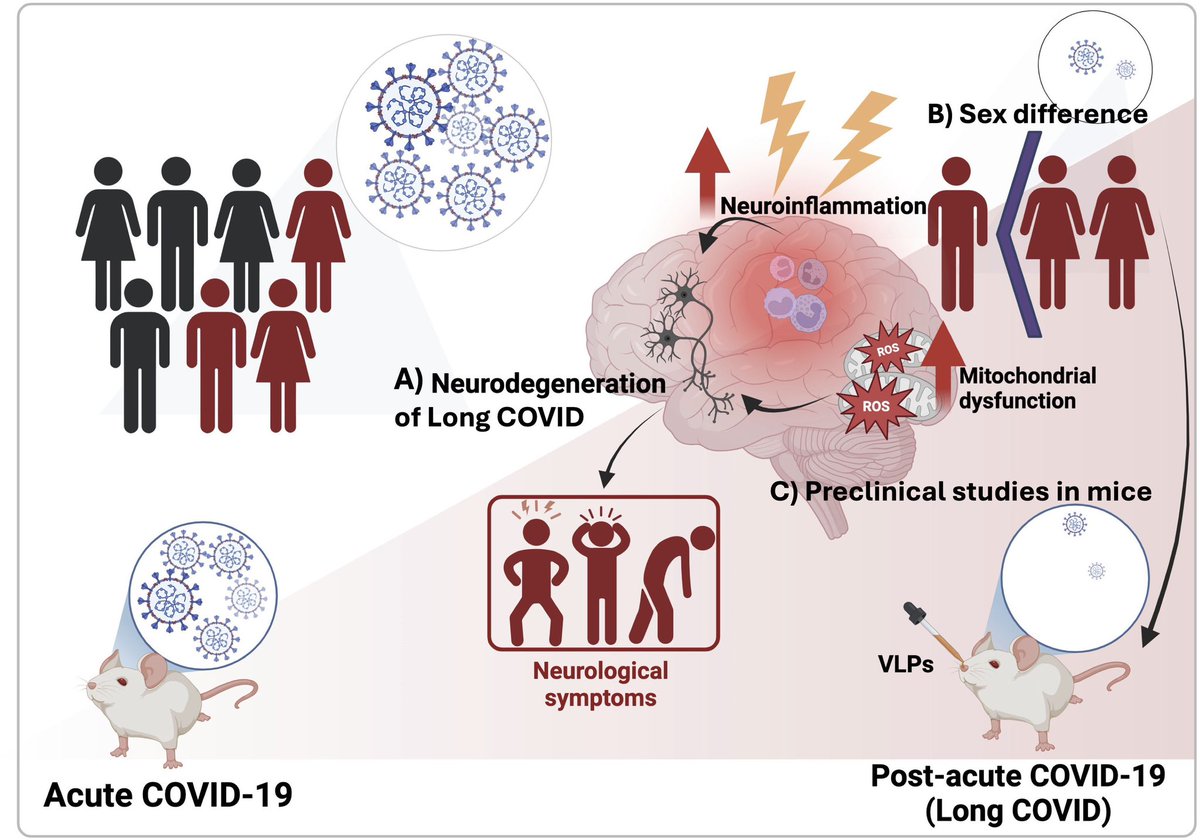
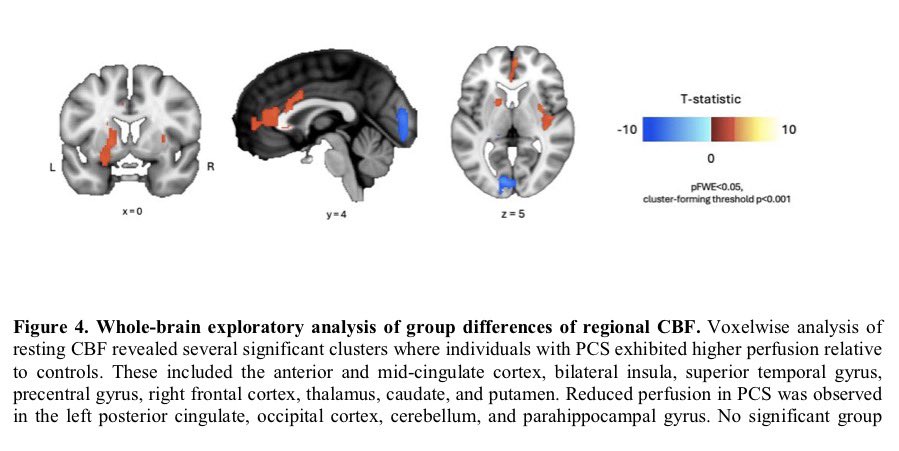
➡️ One year later, women with Long COVID showed clear biological differences — especially signs of gut inflammation and “leakiness.” 2/
➡️ One year later, women with Long COVID showed clear biological differences — especially signs of gut inflammation and “leakiness.” 2/

The study also found anemia and hormone imbalances.
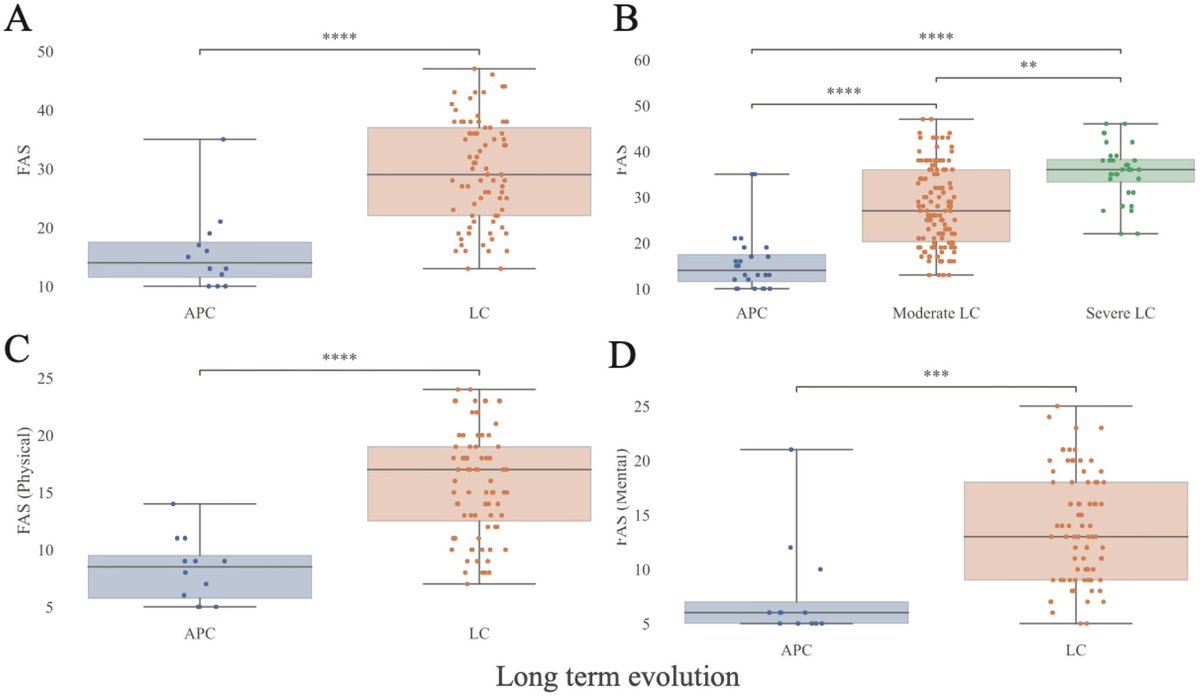
Women with LongCOVID had lower testosterone — a hormone that normally helps control inflammation.
➡️ Lower testosterone was linked to more fatigue, pain, brain fog, and depression. 3/
Women with LongCOVID had lower testosterone — a hormone that normally helps control inflammation.
➡️ Lower testosterone was linked to more fatigue, pain, brain fog, and depression. 3/

So, Long COVID in women may stem from a mix of:
• Gut inflammation
• Chronic immune activation
• Hormone disruption
➡️ Researchers suggest future treatments may include anti-inflammatory therapy, anemia management, or hormone support. 4/
#LongCOVID #WomensHealth
• Gut inflammation
• Chronic immune activation
• Hormone disruption
➡️ Researchers suggest future treatments may include anti-inflammatory therapy, anemia management, or hormone support. 4/
#LongCOVID #WomensHealth

So, LongCOVID in women may stem from a mix of:
• Gut inflammation
• Chronic immune activation
• Hormone disruption
➡️ Researchers suggest future treatments may include anti-inflammatory therapy, anemia management, or hormone support. 5/
• Gut inflammation
• Chronic immune activation
• Hormone disruption
➡️ Researchers suggest future treatments may include anti-inflammatory therapy, anemia management, or hormone support. 5/

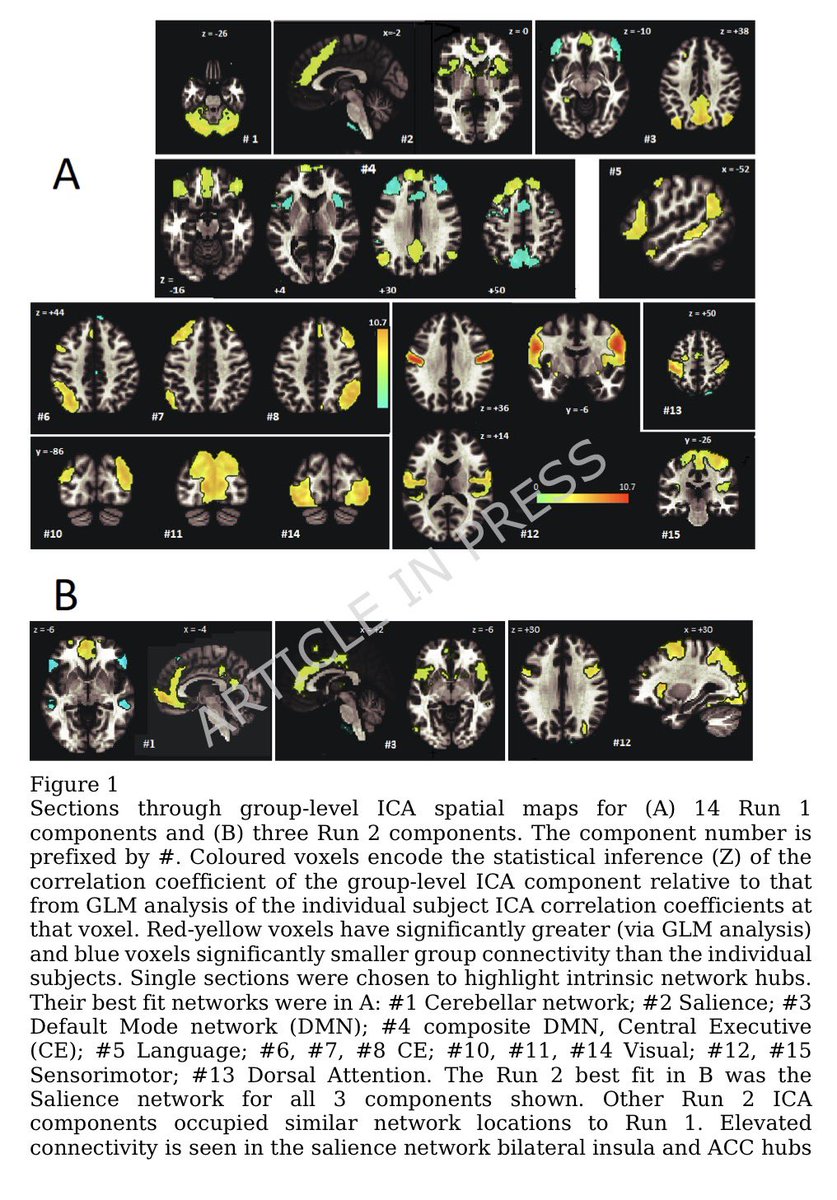
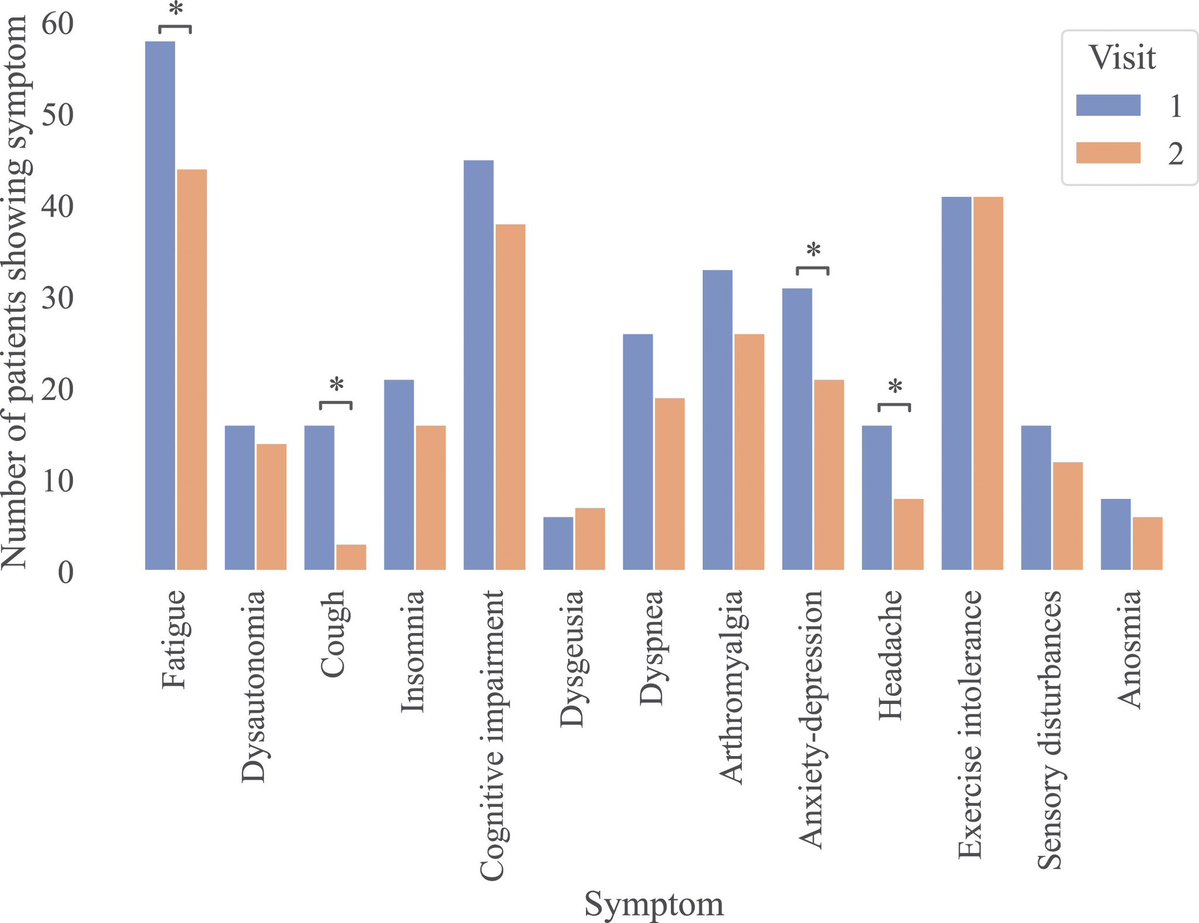
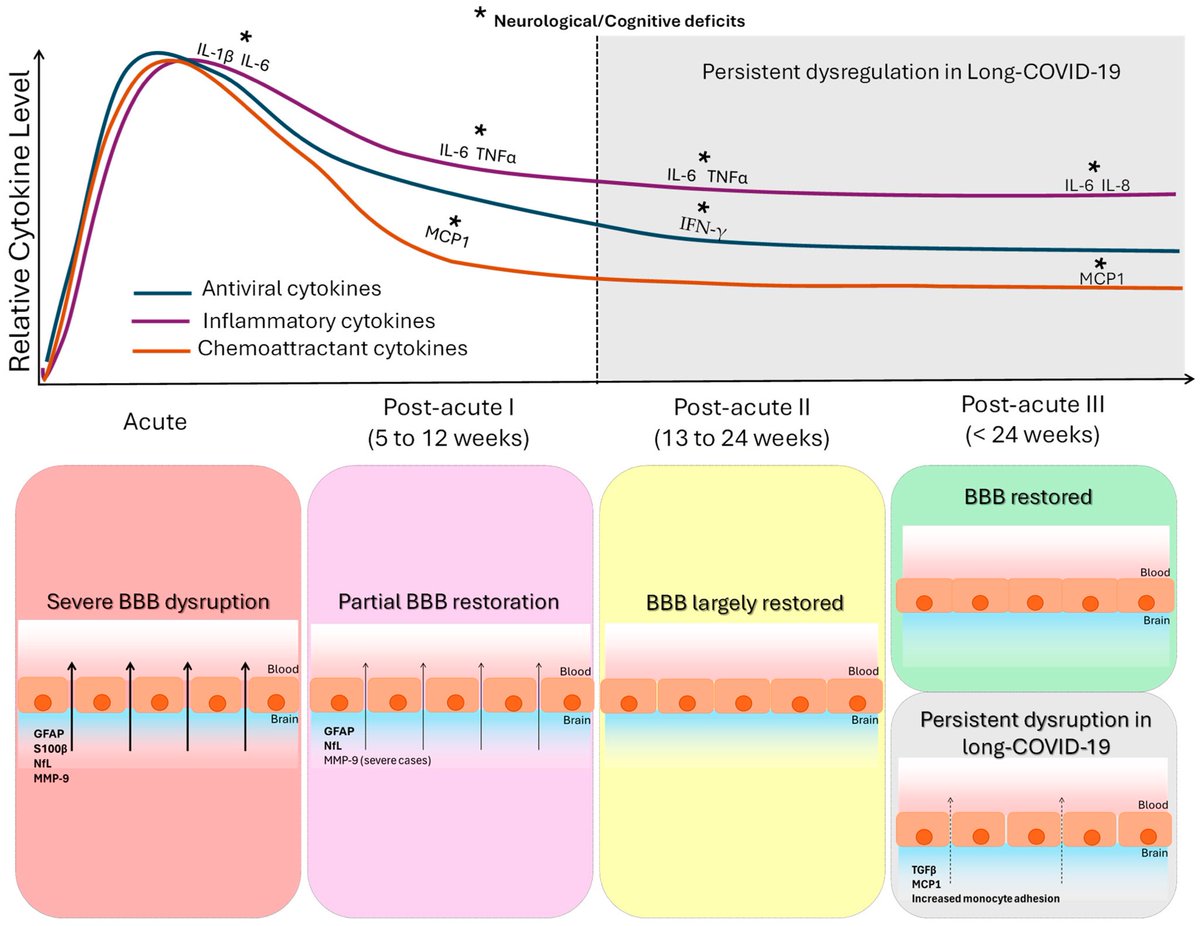
.Transcriptomic data reveal neuroinflammatory signatures in LCF, potentially explaining cognitive symptoms.
We also identify biomarkers that distinguish LCF from LCM and correlate with sex-specific clinical symptoms.
➡️ Overall, LC with ME/CFS is characterized by sex-specific immune, hormonal, and transcriptional alterations, with females exhibiting more severe inflammation. These insights underscore the need for sex-tailored interventions, including consideration of hormone replacement therapy. 6/6
cell.com/cell-reports-m…
We also identify biomarkers that distinguish LCF from LCM and correlate with sex-specific clinical symptoms.
➡️ Overall, LC with ME/CFS is characterized by sex-specific immune, hormonal, and transcriptional alterations, with females exhibiting more severe inflammation. These insights underscore the need for sex-tailored interventions, including consideration of hormone replacement therapy. 6/6
cell.com/cell-reports-m…

• • •
Missing some Tweet in this thread? You can try to
force a refresh