🧵 1/10 We are excited to share our preprint examining pre-pandemic POTS and Long COVID, using deep analysis of the insoluble microclot fraction of blood. Our study shows that the key pathology lies not in protein levels, but in post-translational modifications (PTMs) hidden within fibrinaloid microclot complexes (FMCs). With @Renata_MBooyens, Satish Raj, @dbkell and others. Funded by @POTSActivist
biorxiv.org/content/10.648…
biorxiv.org/content/10.648…
🧵 2/10 Why pre-pandemic POTS matters:
A unique strength of this study is the inclusion of POTS samples collected before the COVID-19 pandemic in the Raj lab. This allowed us to define the baseline molecular signature in controls and classical POTS (entirely independent of SARS-CoV-2); and to distinguish which molecular features in LC-POTS are similar to POTS biology versus acquired through Long COVID.
A unique strength of this study is the inclusion of POTS samples collected before the COVID-19 pandemic in the Raj lab. This allowed us to define the baseline molecular signature in controls and classical POTS (entirely independent of SARS-CoV-2); and to distinguish which molecular features in LC-POTS are similar to POTS biology versus acquired through Long COVID.
🧵 3/10 What we measured:
We quantified fibrinaloid microclot complexes (FMCs) using fluorescence imaging flow cytometry, then performed deep proteomics on the insoluble FMC fraction using LC-MS/MS.
This allowed us to move beyond how much protein is present → to how proteins are post-translationally modified.
We quantified fibrinaloid microclot complexes (FMCs) using fluorescence imaging flow cytometry, then performed deep proteomics on the insoluble FMC fraction using LC-MS/MS.
This allowed us to move beyond how much protein is present → to how proteins are post-translationally modified.
🧵 4/10 Key insight:
At the protein level, differences between groups were minimal. At the PTM (post-translational modification) level, differences were extensive, disease-specific, and biologically meaningful.
At the protein level, differences between groups were minimal. At the PTM (post-translational modification) level, differences were extensive, disease-specific, and biologically meaningful.
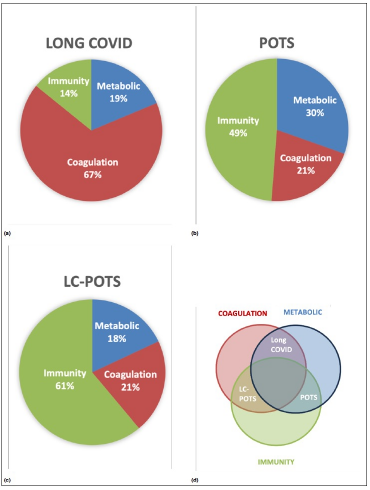
🧵 5/10 Long COVID FMCs showed dominant coagulation pathology:
• Extensive AGE- and oxidation-based PTMs on fibrinogen
• Strong amyloidogenic signatures
• Patterns resembling diabetic glycation
➡️ Consistent with microvascular damage (thrombotic endothelialitis)
• Extensive AGE- and oxidation-based PTMs on fibrinogen
• Strong amyloidogenic signatures
• Patterns resembling diabetic glycation
➡️ Consistent with microvascular damage (thrombotic endothelialitis)
6/10 Pre-pandemic POTS showed a very different signature:
• Prominent immune and complement PTMs
• Oxidised apolipoproteins (apoA1, apoB)
• Relatively limited fibrinogen modification
➡️ An intrinsic POTS biology, independent of SARS-CoV-2.
• Prominent immune and complement PTMs
• Oxidised apolipoproteins (apoA1, apoB)
• Relatively limited fibrinogen modification
➡️ An intrinsic POTS biology, independent of SARS-CoV-2.
🧵 7/10 Long COVID–POTS displayed a hybrid molecular phenotype:
• Coagulation PTMs resembling Long COVID
• Immune PTMs resembling classical POTS
➡️ Providing a molecular explanation for why LC-POTS behaves differently from either condition alone.
• Coagulation PTMs resembling Long COVID
• Immune PTMs resembling classical POTS
➡️ Providing a molecular explanation for why LC-POTS behaves differently from either condition alone.
🧵 8/10 Why PTMs matter:
Many dysregulated peptides were highly amyloidogenic, supporting FMCs as β-sheet-rich, fibrinolysis-resistant aggregates.
Crucially, these PTM signatures are invisible to standard soluble plasma assays.
Many dysregulated peptides were highly amyloidogenic, supporting FMCs as β-sheet-rich, fibrinolysis-resistant aggregates.
Crucially, these PTM signatures are invisible to standard soluble plasma assays.
🧵 9/10 Clinical relevance:
PTM profiling within FMCs opens new avenues for:
• Biomarker-driven diagnosis
• Patient stratification
• Targeted therapies addressing glycation, oxidative stress, and complement activation
This is mechanism-based medicine, not symptom-based.
PTM profiling within FMCs opens new avenues for:
• Biomarker-driven diagnosis
• Patient stratification
• Targeted therapies addressing glycation, oxidative stress, and complement activation
This is mechanism-based medicine, not symptom-based.
🧵 10/10 Take-home message
Long COVID, POTS, and LC-POTS are biochemically distinct diseases.
Their differences are encoded in post-translational modifications inside fibrinaloid microclot complexes; not in protein abundance.
Long COVID, POTS, and LC-POTS are biochemically distinct diseases.
Their differences are encoded in post-translational modifications inside fibrinaloid microclot complexes; not in protein abundance.
• • •
Missing some Tweet in this thread? You can try to
force a refresh






