Long-term cardiac effects of COVID-19 in children:
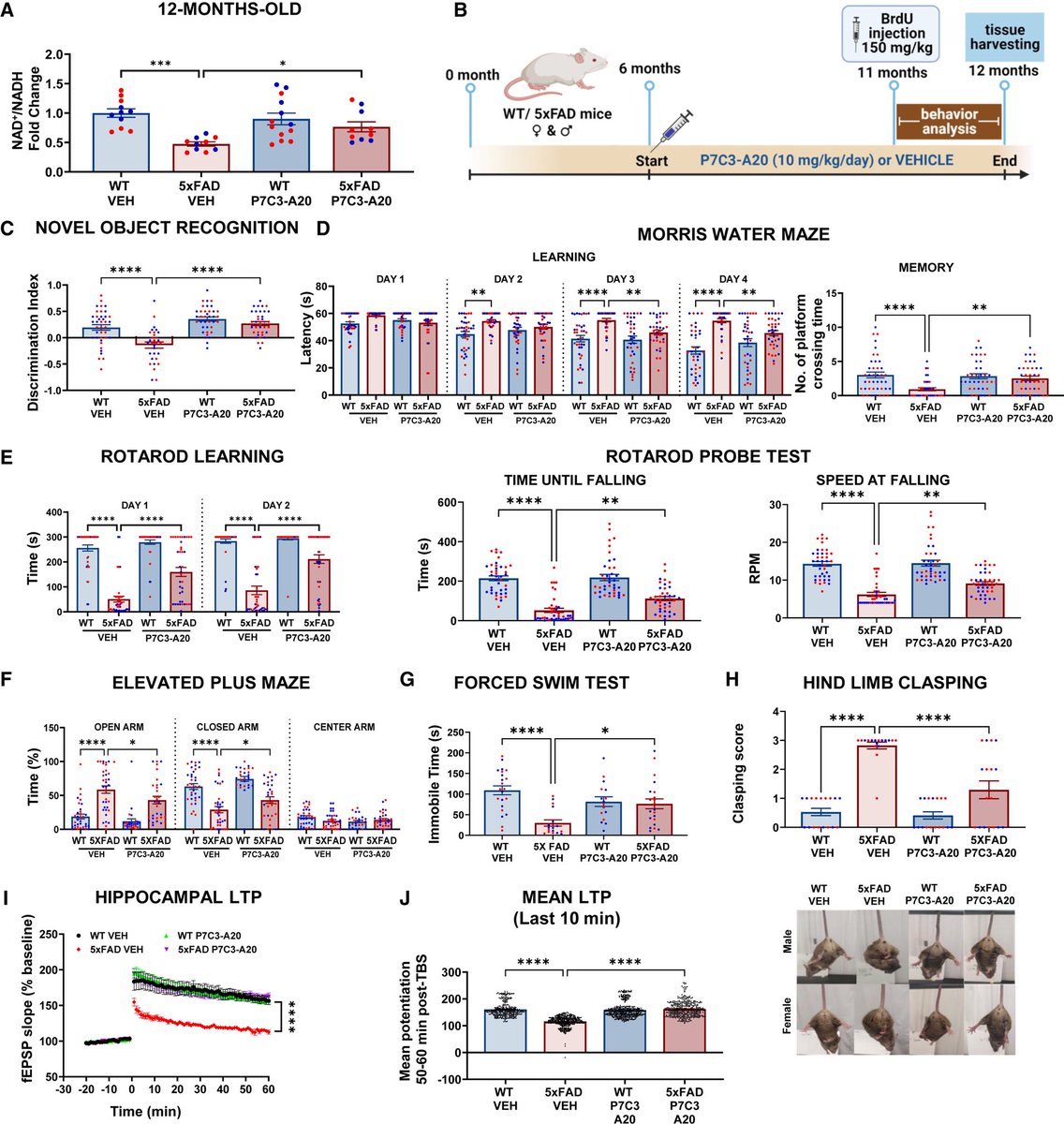
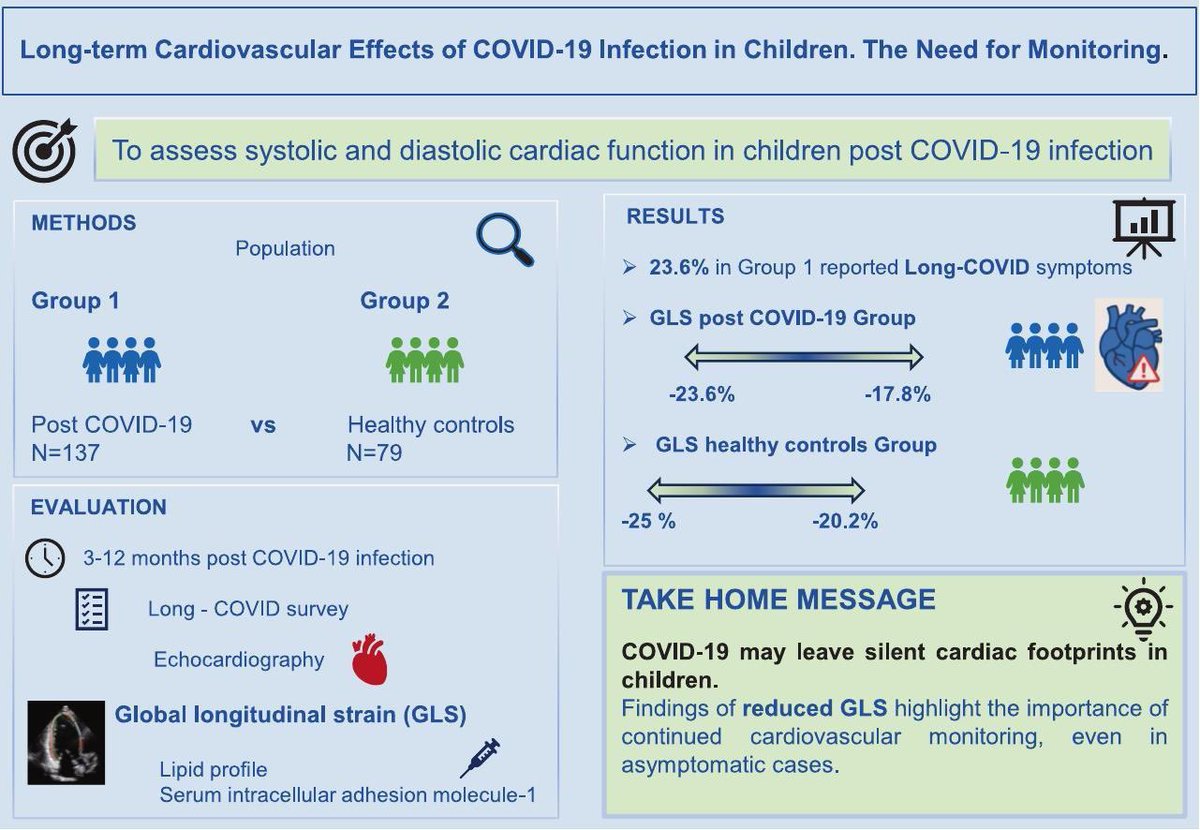
➡️ In a prospective case–control study, children with prior SARS-CoV-2 infection showed a persistent reduction in left ventricular global longitudinal strain (GLS) up to 1 year post-infection.
➡️ Key insight:
Despite normal conventional echocardiographic parameters, GLS was significantly reduced — indicating subclinical myocardial dysfunction that would otherwise be missed on routine echo. 1/
➡️ In a prospective case–control study, children with prior SARS-CoV-2 infection showed a persistent reduction in left ventricular global longitudinal strain (GLS) up to 1 year post-infection.
➡️ Key insight:
Despite normal conventional echocardiographic parameters, GLS was significantly reduced — indicating subclinical myocardial dysfunction that would otherwise be missed on routine echo. 1/
➡️ Severity matters:
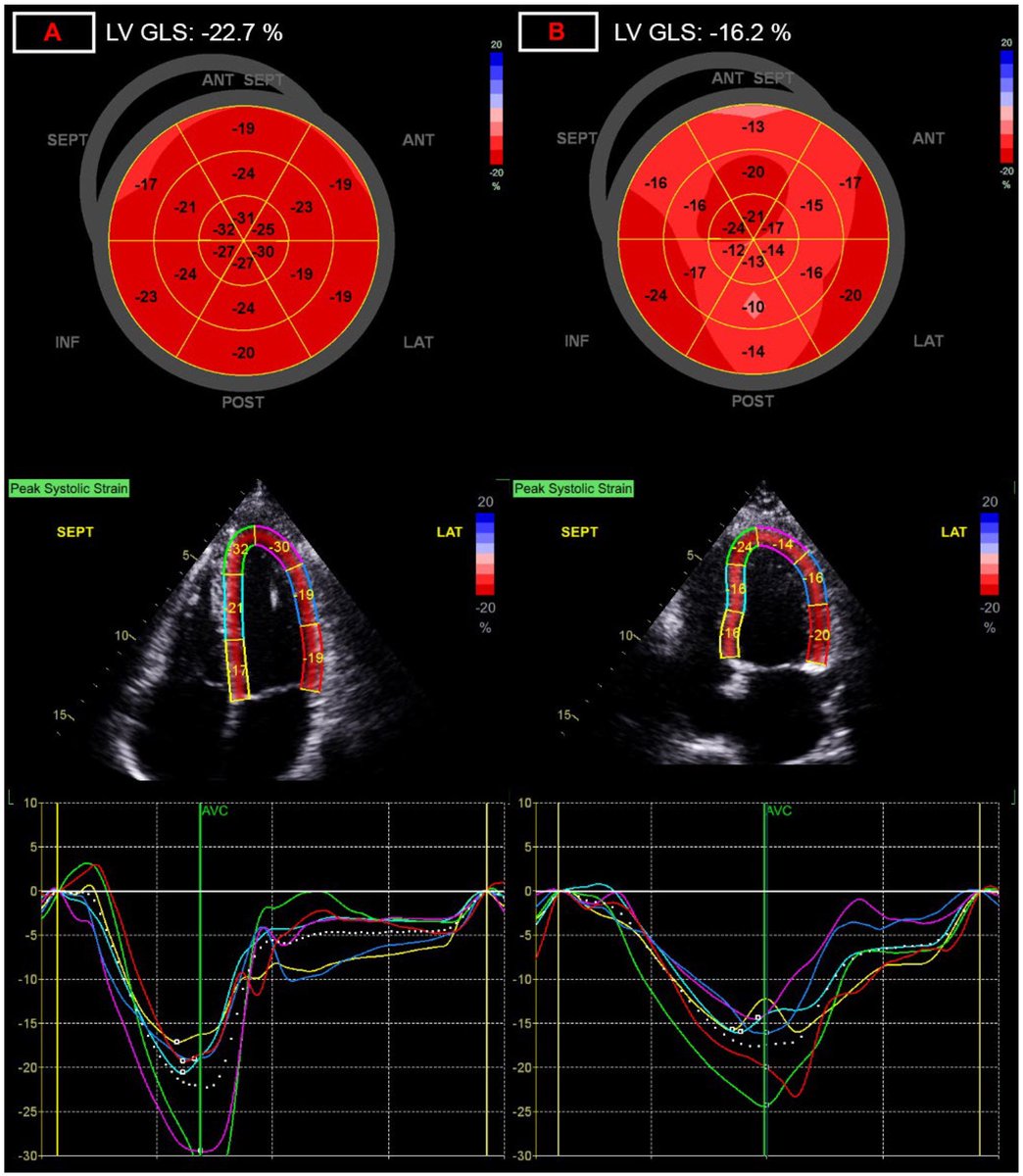
The reduction in GLS was most pronounced in children with moderate to severe acute COVID-19, suggesting a severity-dependent relationship between infection and post-infectious myocardial changes. 2/
The reduction in GLS was most pronounced in children with moderate to severe acute COVID-19, suggesting a severity-dependent relationship between infection and post-infectious myocardial changes. 2/
Beyond the heart muscle:
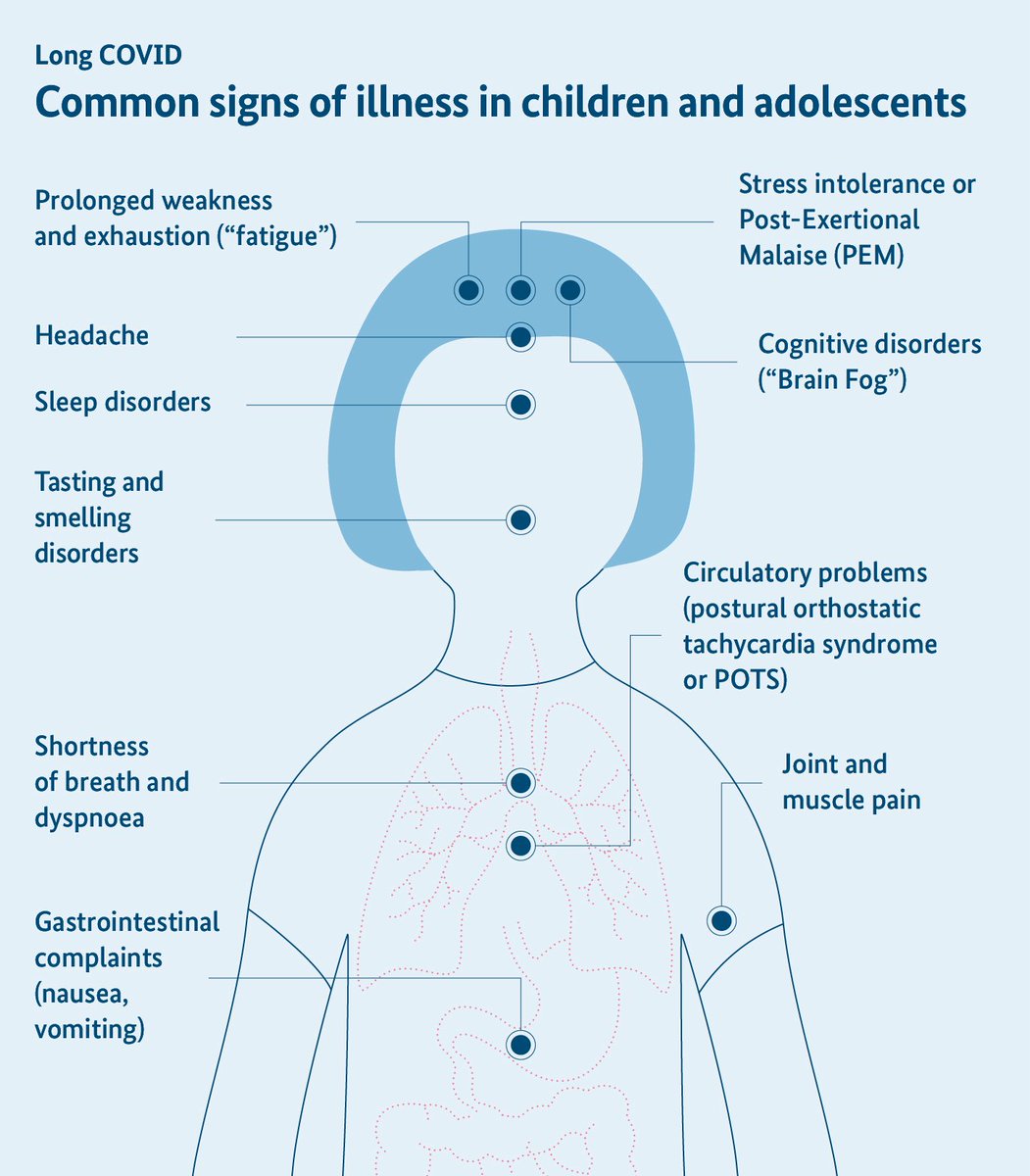
➡️ Nearly 24% of children reported #LongCOVID symptoms (fatigue most common). Elevated sICAM-1 levels in moderate–severe cases point toward persistent endothelial activation.
➡️ This study supports the need for long-term cardiovascular surveillance in pediatric COVID-19 survivors. 3/3
sciencedirect.com/science/articl…
➡️ Nearly 24% of children reported #LongCOVID symptoms (fatigue most common). Elevated sICAM-1 levels in moderate–severe cases point toward persistent endothelial activation.
➡️ This study supports the need for long-term cardiovascular surveillance in pediatric COVID-19 survivors. 3/3
sciencedirect.com/science/articl…
• • •
Missing some Tweet in this thread? You can try to
force a refresh