Long COVID in children is often reduced to fatigue.
This study shows something far more concrete - measurable impairment in language skills - speaking, listening, and reading🧵
This study shows something far more concrete - measurable impairment in language skills - speaking, listening, and reading🧵
https://twitter.com/harryspoelstra/status/1905914509711094070
Study design.
N = 1,244 children (ages 3–18) in Hong Kong.
Three groups.
1. long COVID
2. COVID, fully recovered
3. never infected
N = 1,244 children (ages 3–18) in Hong Kong.
Three groups.
1. long COVID
2. COVID, fully recovered
3. never infected
Language was assessed using LEAP-Q, covering
speaking, listening, reading, writing - in two languages (Chinese + English).
speaking, listening, reading, writing - in two languages (Chinese + English).
Main finding.
Children with long COVID had significantly lower overall language proficiency than both recovered children and never-infected peers.
Children with long COVID had significantly lower overall language proficiency than both recovered children and never-infected peers.
Crucial detail.
There was no significant difference between COVID without long COVID and no-COVID groups.
This directly challenges the claim that deficits are just due to lockdowns, masks, school disruption etc
There was no significant difference between COVID without long COVID and no-COVID groups.
This directly challenges the claim that deficits are just due to lockdowns, masks, school disruption etc
Which domains were most affected?
The largest drops were in speaking and listening.
Reading was also reduced.
Writing showed less consistent differences.
The largest drops were in speaking and listening.
Reading was also reduced.
Writing showed less consistent differences.
Age matters.
No clear effect in kindergarten.
Clear deficits in primary school.
Strongest impact in secondary school students
No clear effect in kindergarten.
Clear deficits in primary school.
Strongest impact in secondary school students
Why this makes sense?
Older students are in the reading to learn phase. Language becomes the tool for complex thinking.
COVID often affects attention, working memory, and cognitive endurance - language performance suffers first.
Older students are in the reading to learn phase. Language becomes the tool for complex thinking.
COVID often affects attention, working memory, and cognitive endurance - language performance suffers first.
Important nuance.
The negative effect appeared in both the first language and English.
This was not just missed school English, but a broader cognitive impact.
The negative effect appeared in both the first language and English.
This was not just missed school English, but a broader cognitive impact.
Limitations (fairly stated)
No pre-infection baseline testing.
Parent-reported measures (not clinical language tests).
Cross-sectional design.
Still, the pattern is consistent and developmentally plausible.
No pre-infection baseline testing.
Parent-reported measures (not clinical language tests).
Cross-sectional design.
Still, the pattern is consistent and developmentally plausible.
What this shows in real life?
Long COVID in children is not subjective or trivial.
It can mean measurable disruption of communication, learning, and daily functioning.
Long COVID in children is not subjective or trivial.
It can mean measurable disruption of communication, learning, and daily functioning.
Estimated Long COVID prevalence in children (RECOVER / JAMA)?
Using the stricter PASC-probable definition
Ages 6–11 ~20%
Ages 12–17 ~14%
These are not marginal numbers!
Using the stricter PASC-probable definition
Ages 6–11 ~20%
Ages 12–17 ~14%
These are not marginal numbers!
And in the United States?
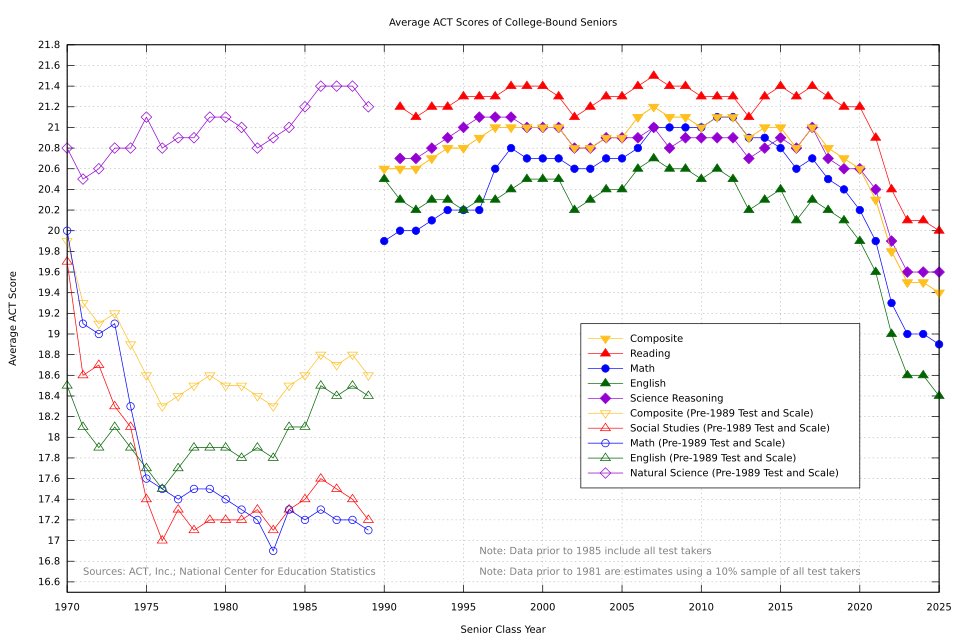
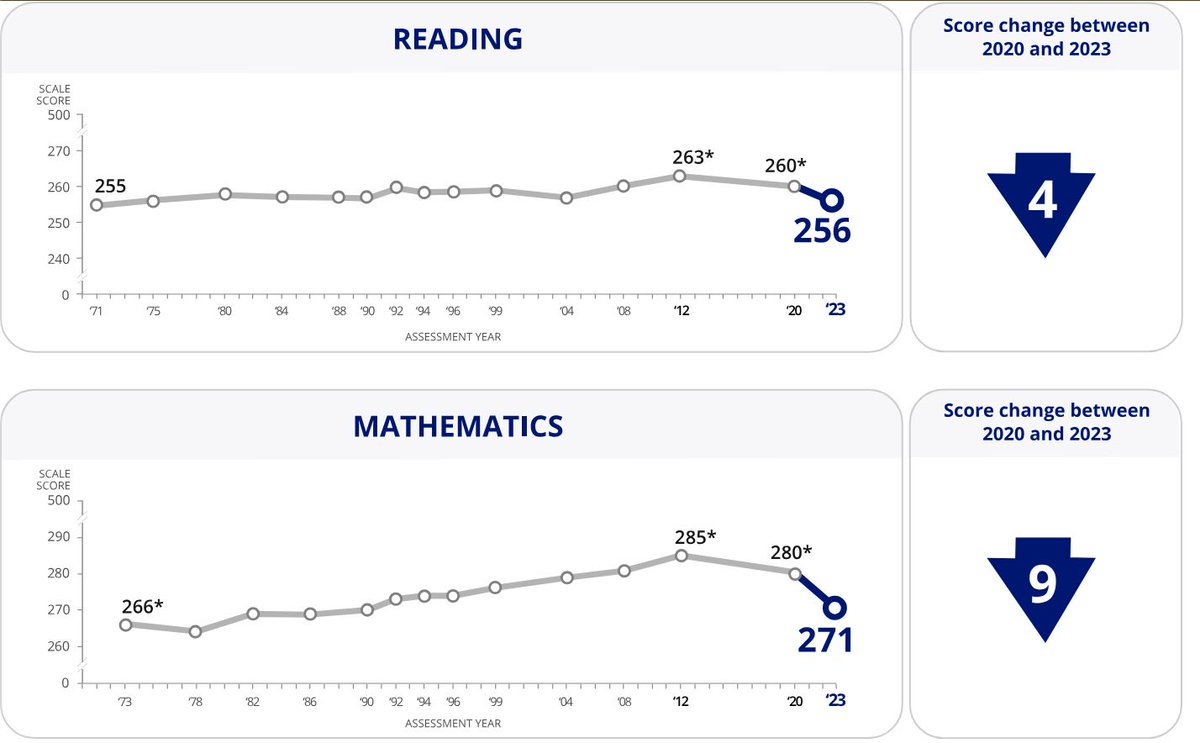
ACT scores were stable for ~30 years.
After 2019, the composite score drops sharply - to the lowest level in decades.
The decline is synchronous across math, reading, English, and science. And persists.
This is not normal fluctuation.

ACT scores were stable for ~30 years.
After 2019, the composite score drops sharply - to the lowest level in decades.
The decline is synchronous across math, reading, English, and science. And persists.
This is not normal fluctuation.
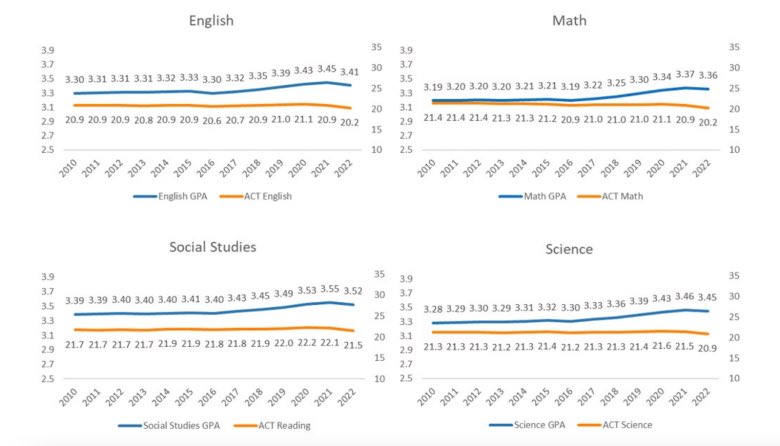
At the same time, high school GPAs rise or remain stable while ACT scores fall.
That divergence matters - grades adapt, standardized tests don’t.
What’s declining is measured cognitive performance, not just grading standards.
That divergence matters - grades adapt, standardized tests don’t.
What’s declining is measured cognitive performance, not just grading standards.
Most countries lack national screening for pediatric long COVID.
Education and healthcare remain siloed.
Recognizing a biological driver would imply responsibility, cost, and abandoning the claim that children are fine.
Instead, the focus stays on learning loss and pandemic disruption -
not on population-level biological burden.
Education and healthcare remain siloed.
Recognizing a biological driver would imply responsibility, cost, and abandoning the claim that children are fine.
Instead, the focus stays on learning loss and pandemic disruption -
not on population-level biological burden.
If public health continues to say children are fine,
it is effectively accepting loss of learning, function, and quality of life in a substantial fraction of a generation. @szupraha @msmtcr @ZdravkoOnline @adamkova_vera @adamvojtech86 @RobertPlaga
it is effectively accepting loss of learning, function, and quality of life in a substantial fraction of a generation. @szupraha @msmtcr @ZdravkoOnline @adamkova_vera @adamvojtech86 @RobertPlaga
A public health call to action.
Stop treating pediatric long COVID as a taboo
monitor cognitive and language outcomes in schools
fund pediatric research and diagnostics
and
most importantly - reduce repeated infections as a preventable risk!
Stop treating pediatric long COVID as a taboo
monitor cognitive and language outcomes in schools
fund pediatric research and diagnostics
and
most importantly - reduce repeated infections as a preventable risk!
Xu at al., The Impact of Long COVID on Language Proficiency Across Different School Levels in Hong Kong. mdpi.com/2076-328X/15/4…
• • •
Missing some Tweet in this thread? You can try to
force a refresh




