
Doctor,MD,Internal Medicine .
Interests: Clinical research,Hobbyist coder, Public Health,
Data science-R,Python,Bayesian inference,old Hindi songs.
How to get URL link on X (Twitter) App

https://twitter.com/prabhud19/status/1403688193183019009Yet Despite 6 months after Bridging Trial and 3 months after Covaxin interim analysis. Companies refuse to publish . Only press release for Covaxin and not even press release for Covishield.

https://twitter.com/sandygrains/status/1345394633195442176Given Oxford Vaccine already approved. It had given us time to run a credible trial and ensure trust. Now with haste , credibility damaged like Vaccines from China n Russia which were given faster approval.
https://twitter.com/anupampom/status/1317704227381071872This is not how we practice Medicine.


https://twitter.com/thesuniljain/status/1285694914131628034?s=20This implies wider spread than previously thought. However Infection fatality rate (Deaths typically taken 7-10 days back after Antibody formation or modelled as log normal distribution) has been estimated at 0.07% (3700 death/ 43-45 lakh affected out of 1.9 crore)..
https://twitter.com/spkalantri/status/12760508044162088972. SOLIDARITY Trial(WH0). No Blinding(open label)

 However when trial results were launched n Coronil Market blitz rolled out. It turned out only ASymptomatic and mild Patients recruited. And no moderate ones . (45 Coronil,50 Placebo). Also they stopped trial at 7 days only.
However when trial results were launched n Coronil Market blitz rolled out. It turned out only ASymptomatic and mild Patients recruited. And no moderate ones . (45 Coronil,50 Placebo). Also they stopped trial at 7 days only.



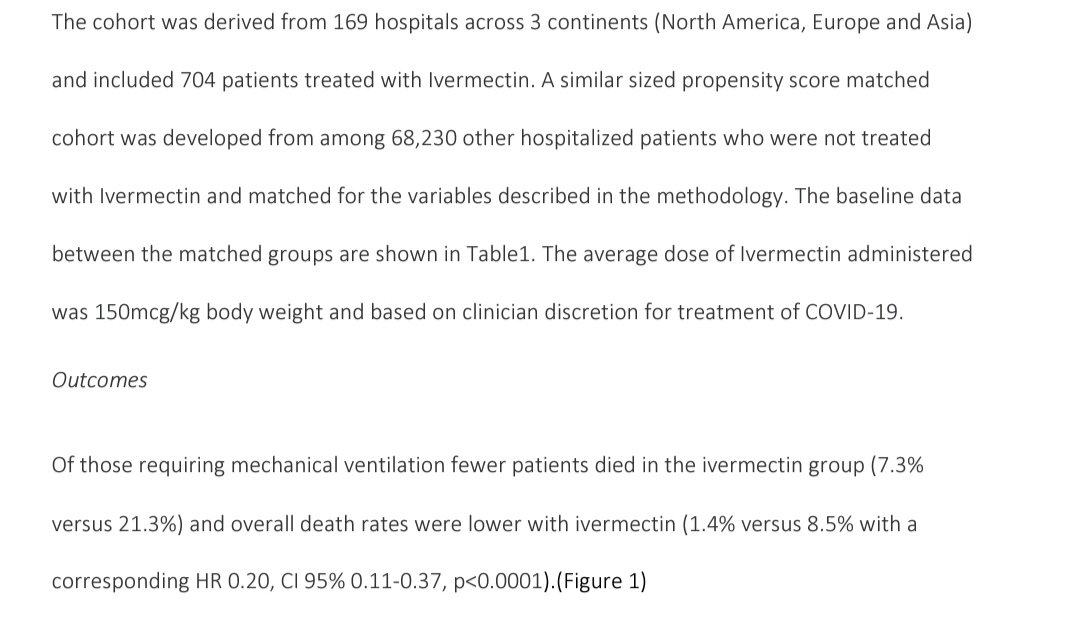
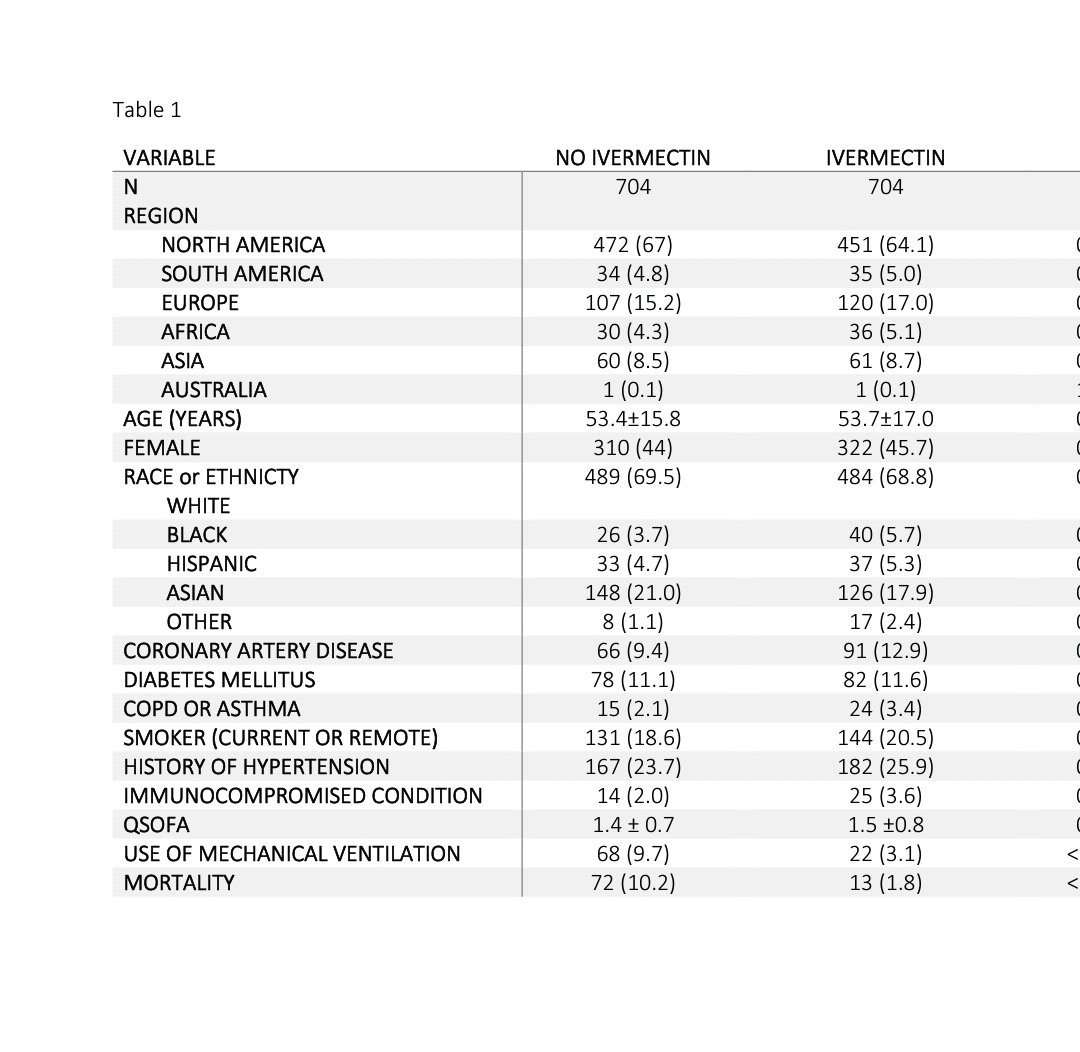 Unless this group at least gives access to data (which they have refused) ,I refuse to trust any analysis from this group. Paper here..
Unless this group at least gives access to data (which they have refused) ,I refuse to trust any analysis from this group. Paper here..
https://twitter.com/DrvanTilburg/status/1249134305441386497Every day hundreds of engineers go inside mines with Protective Equipment (mask/helmets/shields) and do their job.
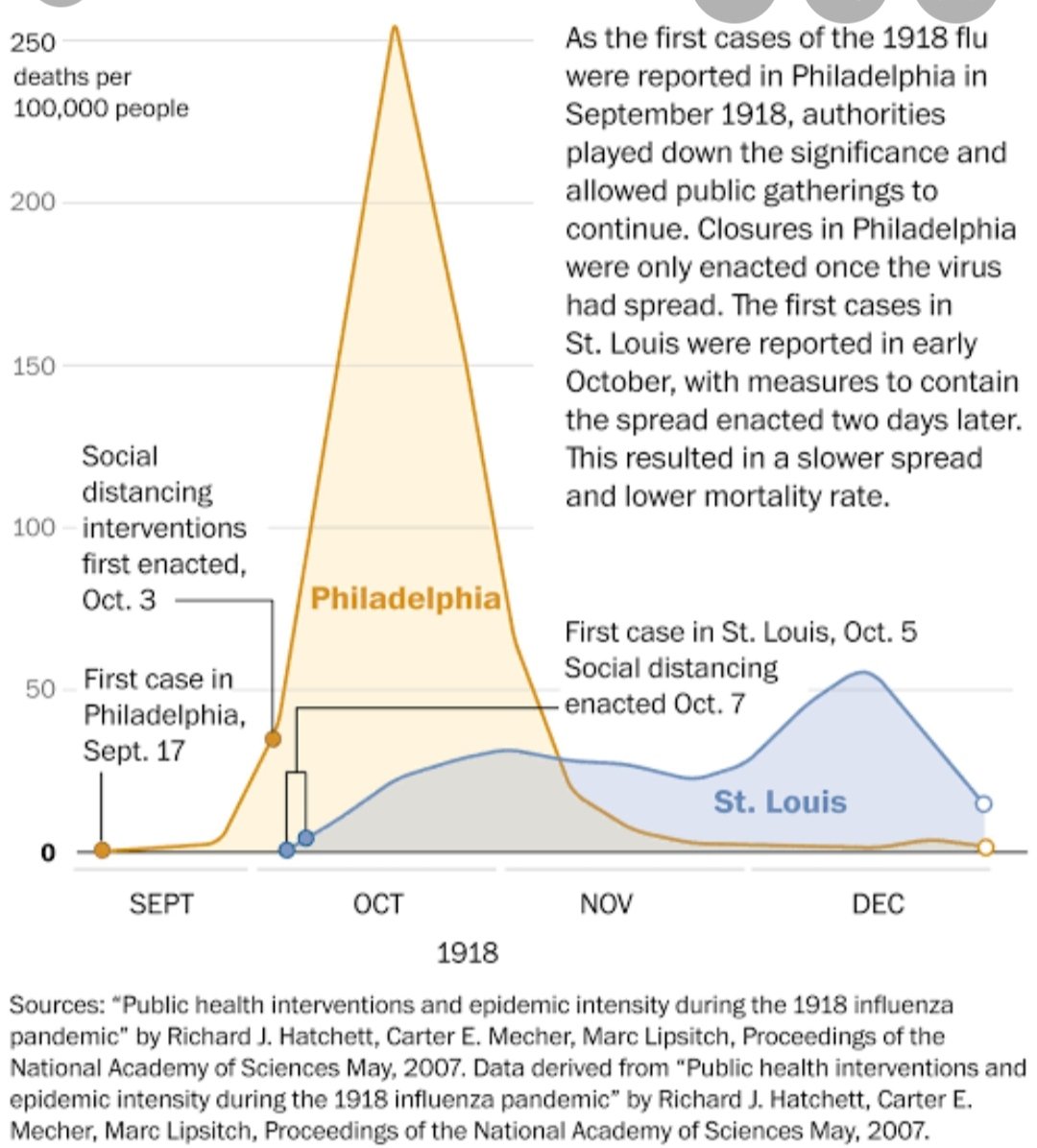
https://twitter.com/drjohnm/status/1247249167510122497?s=20In absence of working treatment ; No Good Antivirals, Vaccine . We are just Fending the Coronavirus Scare. Our Hospitals becoming a Health Care risk due to risk of cross infection.






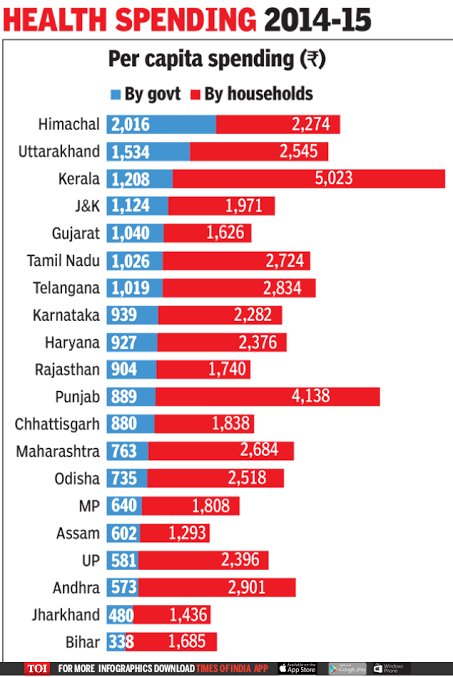
 @DrManojGrover @yates_rob Now Tamil Nadu and Kerala have good health parameters,govt investment and still the OOPE of households are higher than BMARU states, indicating we can't compare apples to oranges.
@DrManojGrover @yates_rob Now Tamil Nadu and Kerala have good health parameters,govt investment and still the OOPE of households are higher than BMARU states, indicating we can't compare apples to oranges.

https://twitter.com/misraudit/status/1047075523933949953@ShamikaRavi @dravirmani The allegation is pretty serious ,considering the survey was overseen by experts from WHO,UNICEF and gates Foundation.