
Biostatistician trying to work out the best ways of evaluating medical tests. Views expressed are my own and do not necessarily reflect my employer or funders.
9 subscribers
How to get URL link on X (Twitter) App






 Long link is here:
Long link is here:




 Remember that the law made kids, teachers, families and bubbles had to isolate for 10 days even when the PCR came back negative, despite the @RoyalStatSoc spending days trying to explain the issues to @DHSCgovuk.
Remember that the law made kids, teachers, families and bubbles had to isolate for 10 days even when the PCR came back negative, despite the @RoyalStatSoc spending days trying to explain the issues to @DHSCgovuk. 

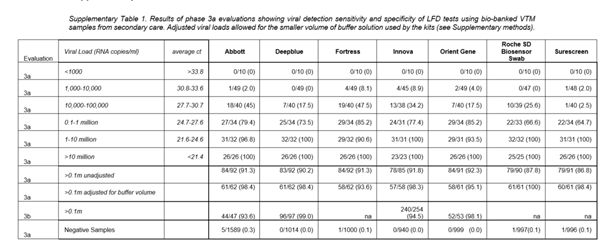
https://twitter.com/deeksj/status/1383168237911871490#1 "the lateral flow test has the remarkable ability of only picking up people who are likely to be infectious, or other words a danger to others."


 The clue to which one is most up to date is on the back page (the one in the box is the out of date version).
The clue to which one is most up to date is on the back page (the one in the box is the out of date version).

https://twitter.com/drdavideyre/status/1379085356700602375Using this test (or better ones) backed with PCR in test-and-trace centres could revolutionize contact tracing- if people get their results and meet with an infection control team before they leave the centre we will be moving everything forward 3 days. Add in financial help too.

 What happened in secondary schools?
What happened in secondary schools?



https://twitter.com/CwmTafMorgannwg/status/1373961796080562186However, in the whole sample 33,315 LFD tests were completed across 12 centres. Of these, 763 were positive, representing a positivity rate of 2.3%.

 Using the Government figures from @ab4scambs conservativehome.com/platform/2021/…
Using the Government figures from @ab4scambs conservativehome.com/platform/2021/…