
My very first senior author manuscript. Equally important: My very first preprint! So stoked to finally be a direct participant in this long overdue process for accelerating biology.
Actin chromobody imaging reveals sub-organellar actin dynamics biorxiv.org/content/10.110…
Actin chromobody imaging reveals sub-organellar actin dynamics biorxiv.org/content/10.110…

Fair warning: If you don’t care about actin, organelles, or imaging, you may want to mute this thread. The rest of you kids should buckle up, cuz I’m gonna take you on a little journey. Will update this thread when I am able.
Ok I'm back. Since I like to consider twitter dot com sort of the wild wild west of ideas, I'm going to be a bit more philosophical/speculative than I was in the paper. Ironically, there may be more detailed background and discussion here than in the manuscript!
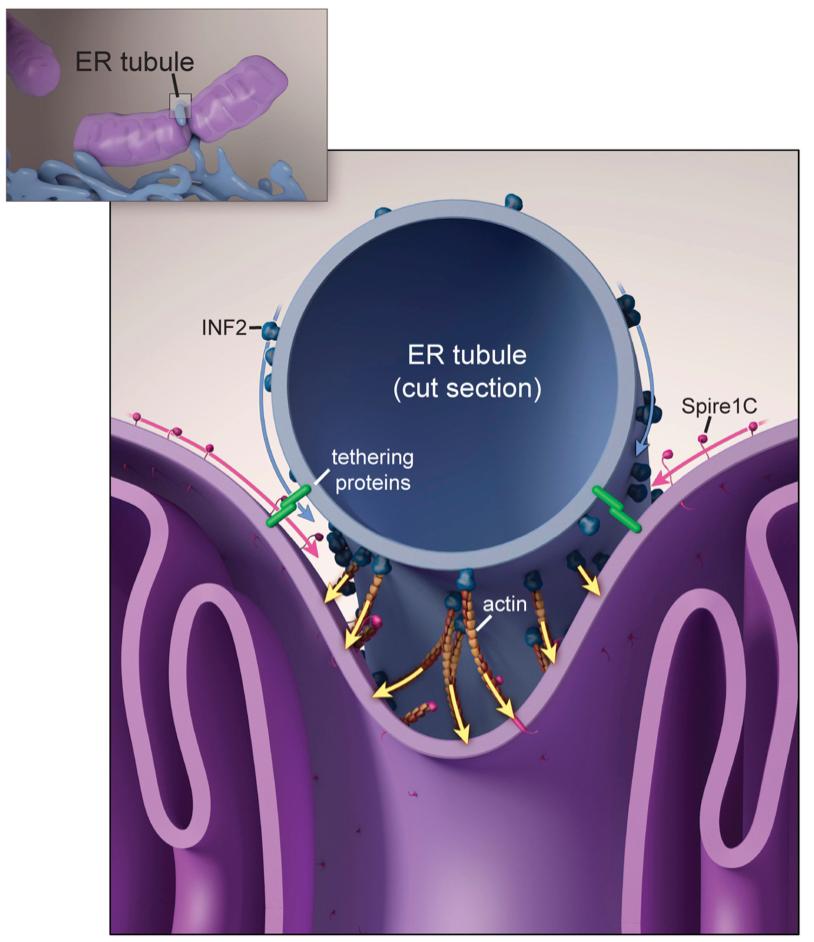
First, I would like to set the stage with this landmark paper by @FriedmanLabUTSW with the @jodi_nunnari and Voeltz lab, which reported something remarkable: The ER forms tubules that wrap around and constrict mitochondria prior to Drp1-mediated fission.
ncbi.nlm.nih.gov/pmc/articles/P…
ncbi.nlm.nih.gov/pmc/articles/P…

Then @hhiggslab showed the ER-anchored formin protein INF2 directly participates in this process - INF2 KD lowers mito fission, dominant active INF2 increases mito fission. This is important because it isn't obvious how 50nm ER tubules could constrict mitos. But actin can! 

Prolific as ever, the legendary @hhiggslab next showed similar results for myosin II, an actin-tensioning, force-generating badass motor protein. Green is MyoII, Red is Mitotracker.
doi.org/10.1016/j.cub.…
doi.org/10.1016/j.cub.…
Then while doing my postdoc in the @JLS_Lab, we showed that the formin-binding, actin-nucleating protein Spire has a unique splice isoform with a mito outer membrane targeting sequence that similarly modulates mito fission. Leading to this model here.
elifesciences.org/articles/08828
elifesciences.org/articles/08828

Essentially, Spire, INF2, and who knows what else helps drive actin accumulation *specifically* at ER-mito intersections. I think this is a really nifty model and am obsessed with testing it and refining it further. But there's a problem.
While I could show that overexpressing Spire induces actin accumulation on the surface of mitos, I never managed to get any images (live or fixed) that convincingly showed actin accumulation specifically at ER-mito contacts. And believe me, I tried.
Meanwhile other papers from @hhiggslab (who at this point I am convinced is not of this world), as well as gorgeous work from @aaandmoore and the Karbowski lab (whose paper preceded mine, actually) provided additional insight towards the role of actin in mito fission. 

This @hhiggslab video shows the most clear live cell imaging of actin accumulating at a mito fission site to date. Clearly actin is accumulating at or near fission sites. But is actin directly attached to mitos or the ER? Need more resolution! elifesciences.org/articles/11553
Also - this is with ionomycin treatment, which may lead meanie jerks like @OdedRechavi to ask "what is the physiological relevance?".
Anyways, I still struggled to capture any obvious accumulation of actin on mitos or ER, even with phalloidin, the "gold standard" of actin labeling. Here's are examples of what mito or ER vs phalloidin staining looks like in my hands. 



If you squint, you could perhaps convince yourself that there are regions of association. But how can you be sure this isn't due to resolution limitations? Without sufficient resolution, every single person on Twitter tonight is "colocalized" on Earth. Need. More. Resolution.
After seeing Grosse's work (nature.com/articles/ncb36…) using nuclear actin chromobodies (AC), I wondered: What if we tethered actin chromobodies to mito or ER membranes!? So we made constructs with the mito outer membrane targeting sequence from Fis1 fused to ACs (AC-mito).
When I first saw the AC-mito labeling pattern, I was floored. Very distinct subdomains of enrichment that dynamically change, cross over, and wrap around mitos in patterns I never saw with phalloidin staining. Also note the high accumulation in the perinuclear region.
Here's another example of a movie showing AC-mito's tendency to enrich in subdomains on mitochondria.
I was also floored when I saw the localization pattern for AC-ER. Once again - a totally unexpected result based on previous imaging with normal actin probes. Notice that AC-ER - which is tethered exclusively to the ER membrane - clearly accumulates at mito contacts!
Here is another example of AC-ER localization pattern, again showing accumulation at mitos - in some cases you could almost outline the mitos with the AC-ER label.
Ok, so now that I was super duper excited about seeing these patterns, I started to lose sleep over whether or how I could trust these results at face values. BRING IN THE CONTROLS
In all seriousness - I love designing controls. The first thing I worried about was whether perhaps the accumulations we saw were independent of actin - perhaps there are just organelle subdomains that our membrane targeting sequences particularly like to hang out at?!
That's why we did this: Co-express AC-GFP-mito/ER with mCherry-mito/ER. We saw accumulation is indeed dependent on the actin nanobodies (note you need to click on the image to see the whole thing - annoying). 

I really love the lower right image because it looks just like the model I put in my 2015 eLife paper, but could never get a clear image of: Accumulation at ER-mito intersections! 


So now that we've done all the controls, we can move on, amirite?
How else can we show that these sites of enrichment are labeling bona fide F-actin? Well...let's kill the actin! Latrunculin B and Cytochalsin D, along with the internal control of a pan-actin probe, show that depolymerizing the actin destroys virtually all the enrichment. 

Ok. But what if our Fis1 targeting sequence is somehow outcompeting endogenous Fis1?!? That would totally alter mito dynamics, defeating the entire purpose of these efforts!
Rest, my child, for immunofluorescence staining of the N-terminal cytoplasmic side of endogenous Fis1 (not present in our C-terminal tail construct) shows that there is no change in endogenous Fis1 localization in transfected cells. 

Alright alright. So we've shown that the localization pattern is dependent on our actin nanobody, is dependent on F-actin, and doesn't compete with endogenous Fis1. This is the part where some people started to think I was being obsessive.
But great science IS "obsessive" (i.e. careful). One great scientist is my long-time collaborator/friend @_OmQu, who brilliantly suggested we try swapping out the actin nanobodies for LifeAct, and the Fis1 mito targeting sequence for a different one. I said "HELL YES!"
As you can see, swapping either or both the mito targeting or actin-binding domains of our AC-mito probe yields similar results. 

Ok great - so we can confirm that whether it is Fis1 or some other mito targeting sequence, or whether it is nanobodies or LifeAct, we see enrichment of actin on mitochondrial subdomains. Doesn't it bother you at all that we don't really see this with pan-actin probes? Why not?!
One key point I try to make clear in our paper is that we have an enormous background signal issue with actin labeling. Actin is *everywhere*, and most of the actin is way more dense and stable than the relatively small and ephemeral actin filaments driving organelle fission.
Nonetheless, if these structures are real, we should still be able to find them using our AC probes as "guides". So we decided to use gold-standard phalloidin staining to test whether we can indeed detect F-actin at these enrichment sites. And that's when we ID'd a major problem.
We found that fixing the cells almost totally destroys the labeling of our AC probes! This is something I'm sure @HenriquesLab, @joachimgoedhart, and others can confirm as a real problem with all FPs. But it isn't just the signal intensity - the distribution is also more diffuse. 



We believe this means these AC-labeled structures are very small/transient. Interestingly, other structures can similarly become diffuse during fixation - @Anders_S_Hansen showed me a @TjianDarzacq paper with similar results for Sox2. elifesciences.org/articles/22280
In spite of massive loss of labeling there were still some enrichment sites in the cell. Although almost everything was less clear after fixation, we could still ID bona fide sites of enrichment by using images of the cells pre-fixation. It wasn't pretty, but...
it gets WORSE!

it gets WORSE!


How/why could it be worse?
Because of the enormous background actin signal in the cell, we needed to jack up the contrast to ridiculous heights to detect phalloidin at AC-labeled sites. This is the only time I've ever been proud to publish such an ugly image.



Because of the enormous background actin signal in the cell, we needed to jack up the contrast to ridiculous heights to detect phalloidin at AC-labeled sites. This is the only time I've ever been proud to publish such an ugly image.

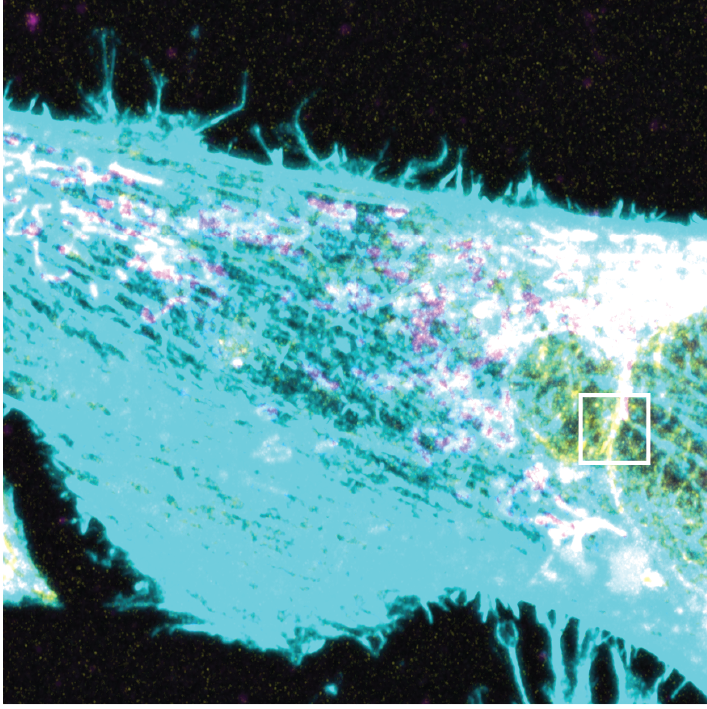


Why be "proud" to show this image? Because it proves the need for advanced probes to image these kinds of subcellular actin structures. It also highlights the importance of live vs fixed cell imaging. Finally, these results help explain why (most) EM doesn't see these structures.
At this point, we've convinced ourselves that the AC probes are labeling real actin structures. Tomorrow, I'll go into the experiments we did to test whether these actin structures have any functional role. Normally I'd continue, but I'm verrrry sleep deprived right now.
So the first obvious question for us was: Do we see ER and/or mito-associated actin at mito fission sites? And, if so, when? So we acquired timelapses of AC-mito/ER and Drp1-mCherry (the GTPase that drives fission, and also happens to bind to and be activated by actin).
One thing we noticed in our movies is AC-mito often appeared to be *parallel* to the axis of fission, which contradicts the cartoon in my 2015 paper. 





But, more importantly, it is *consistent* with gorgeous EM work from the Svitkina lab that just came out.
nature.com/articles/s4155…
nature.com/articles/s4155…
Anyways, we can see that mito- and ER-associated actin accumulates at mito fission sites, often before Drp1 moves in. Note that these movies are not as pretty as some of the other images I showed earlier. That's because we are now using a VERY low expression ubiquitin promoter!
We also imaged AC-mito/ER and the ER, and saw accumulation of ER- or mito-associated actin at ER-marked fission sites.
Reminder: our AC probes are just a ≤4nm nanobody fused to GFP and a ~15aa tail anchor sequence - there's no way the sensor is >10nm from the membrane.
Reminder: our AC probes are just a ≤4nm nanobody fused to GFP and a ~15aa tail anchor sequence - there's no way the sensor is >10nm from the membrane.
Here's some quantification for each of those movies. Note that often the brightest regions were on the ends of the mitos, not the fission sites. Once again, that is consistent with recent EM data from Svitkina's lab. 


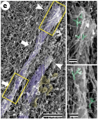
So now I want to mention yet another landmark paper (actually, TWO) from the Voeltz lab, this time showing that ER tubules mark sites of *endosome* fission (doi.org/10.1016/j.cell…) and that the actin-binding protein coronin is involved (doi.org/10.1016/j.cell…). 

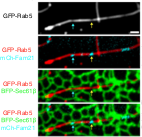

So anyways, given we know that ER-actin drives mito fission, and we know that ER drives endosomal fission, no less with actin regulatory proteins involved, is it crazy to speculate that perhaps ER-actin drives endosomal fission? Seemed reasonable to me...
So we went ahead and imaged AC-ER with the endosomal marker Rab5a. Lo and behold, we found ER-associated actin accumulating at endosomal fission sites.
At this point, I am beginning to get a bit wild-eyed, and I'm wondering: Does the ER regulate *all* organelle fission? More specifically, is ER-associated actin the mechanism by which all organelle fission is driven?!? 

So...yeah. We went ahead and looked at ALL THE ORGANELLES - mitochondria, endosomes, peroxisomes, and lysosomes - alongside AC-ER. 

As you can see, we invariably found AC-ER at each of these organelle's fission sites. EVEN THE FRIGGIN' GOLGI. 

So yeah...I'm convinced that we have uncovered a "universal" mechanism by which the cell regulates organelle fission! It does so through the ER, which provides the scaffold for actin assembly and organization as necessary to provide the forces needed.
Before we move on to the last really exciting observation we made with these probes, I want to talk a bit more about the role of the ER in regulating mitos and I guess apparently all the organelles.
Gorgeous work by @cohenlaboratory and @Valm_Lab while postdocs in the @JLS_Lab showed that the ER is the central node interacting with all the organelles. 



So now I want to take a bit of a detour into what is apparently controversial in some circles, but I think is intellectually thrilling: A paper by @BaumBuzz and @baobabbaum titled "An inside-out origin for the eukaryotic cell."
doi.org/10.1186/s12915…
doi.org/10.1186/s12915…
Classical endosymbiotic theory posits that protomitochondria joined up with our ancestral archael cell via some kind of phagocytic event. Here's what the Baum team says about that. 



So they propose an alternative model, the "inside-out" model, wherein the ancestral archael cell (which eventually becomes the nucleus!) produced protrusions that interacted with neighboring bacterial cells, i.e. protomitochondria. 

Eventually the protrusions grew around the protomitochondria, exchanging lipids, nutrients, etc. I really like this model because it is much less traumatic than engulfment, allowing for gradual refinement and tightening of the relationship over many generations. 

But in addition to being a fun opportunity to think about the evolution of the cell, and challenge what is pretty much considered canon, this also perhaps provides a useful perspective on how/why the ER is interacting with and regulating mitochondria and other organelles!
If you think of the nucleus as the descendant of that ancient archaeal cell, and the ER as extensions/protrusions from the nucleus that were interacting with the protomitochondria, we realize these interactions are *ancient*. Like, long before LECA was a twinkle in LOKI's eye.
Yeah I know that was a really, really nerdy, really really bad joke.
In any case, another key feature of the inside-out model is that those protrusions were formed by actin, which we inherited from our archaeal ancestors. So not only is the interaction between what are now ER and mitos ancient, but actin was also directly involved from the start?!
Anyways, that's all pretty speculative obviously, so I didn't mention it in the paper. But I still think it's an intriguing connection that may very well gain acceptance as new evidence rolls in. But since I mentioned the nucleus, ER, and actin, check this out. 

As you can see, there is a distinct meshwork of actin bundles on the nucleus detected by our AC-ER probe. I've never seen perinuclear actin so clear like this before. Once again - the huge actin filaments throughout the rest of the cell probably mask it. But our EM confirms it.
Here's a fun video of that image so you can see it in 3D. And so we can show off just how pretty these cells are (very important, obviously).
So there you have it folks! An *entire paper*, and some extra background/musings all in one massive tweet thread. I really enjoyed this exercise - I hope it was at least somewhat entertaining for you.
One last note about this work: This was a massive, superhuman effort involving many, many imaging sessions into the 4AM range, often running not two, but THREE microscopes simultaneously (set up one timelapse, go to the next scope to set up another, and around and around).
My postdoc Cara Schiavon and the core light microscopy scientist Tong Zhang both killed it. We also had invaluable help from collaborators @_OmQu and his undergrad @Jasmine_Feng16 (who is coming to work in my lab this summer - yay!) making many of the control constructs.
Robert Grosse and his postdoc Bing Zhao had the chromobody probes and at the time I didn't have funds to buy the plasmids so they made the first constructs for me. Not every day you meet people willing to help out a total stranger with a crazy idea. Science culture is amazing.
• • •
Missing some Tweet in this thread? You can try to
force a refresh




