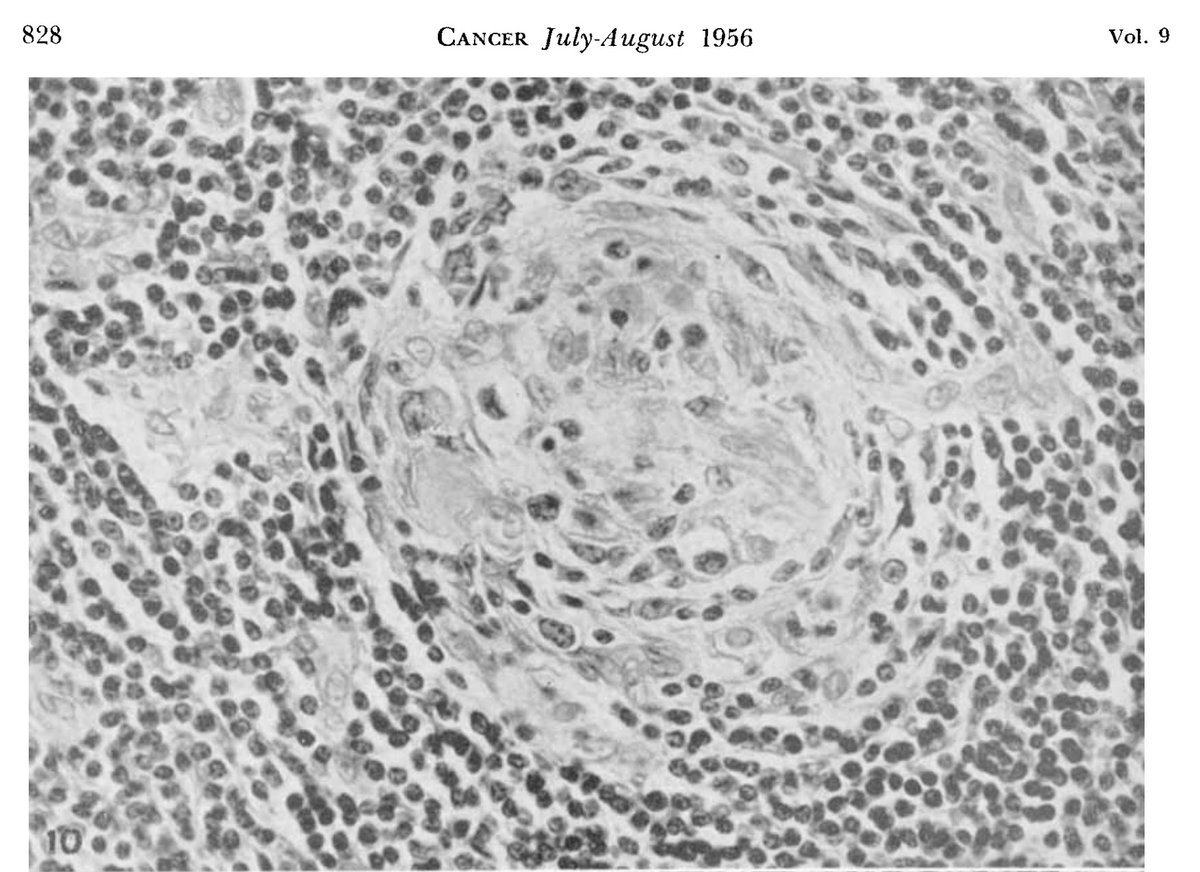
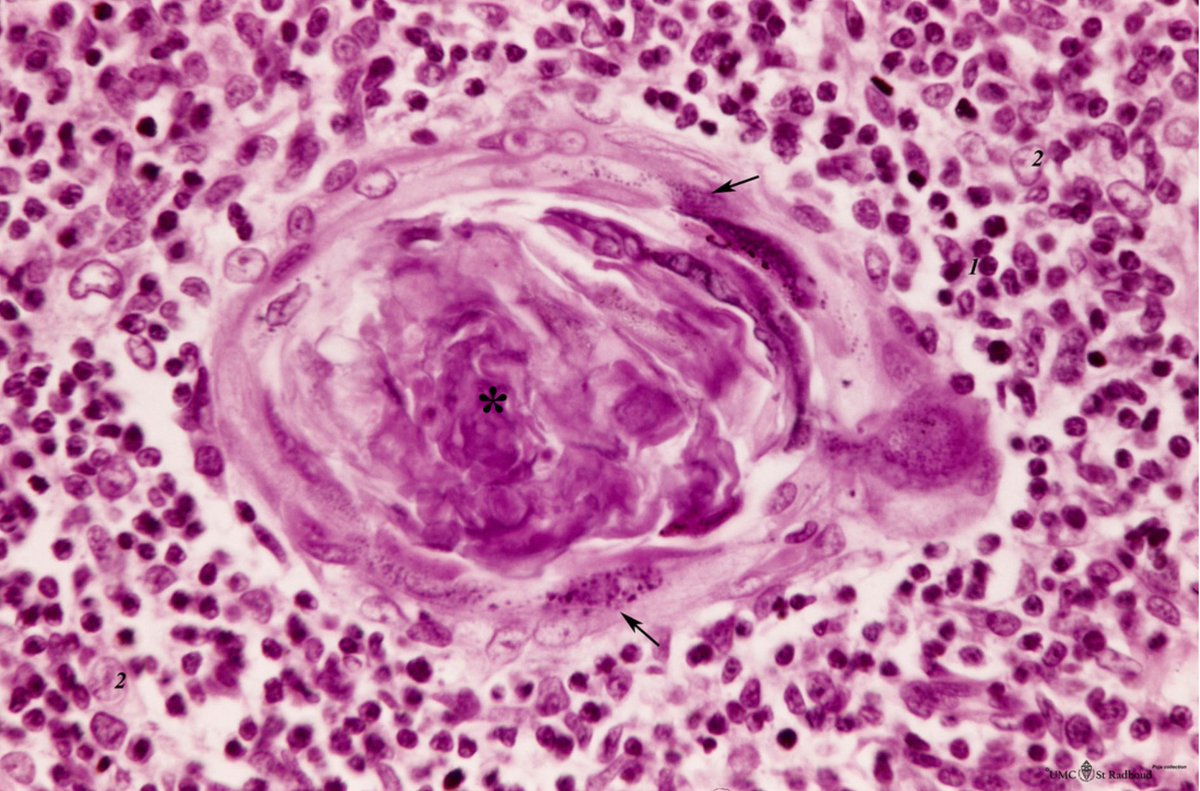
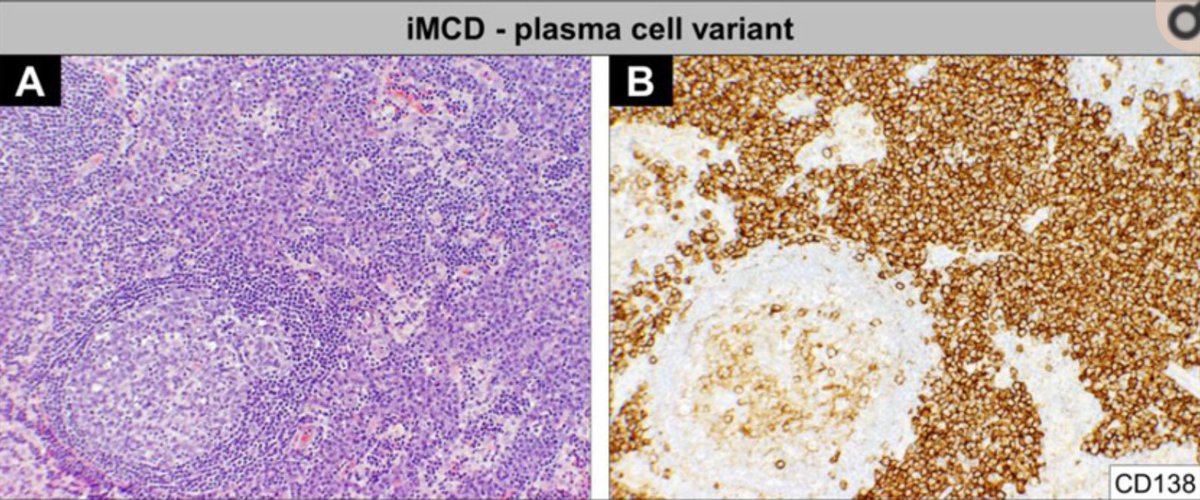
It became clear that there were 2 distinct clinical subtypes of this entity - termed “unicentric”and “multicentric” Castleman’s disease.
Patients with HHV-8 associated MCD seemed to have a higher incidence of non-Hodgkin lymphoma, estimated at 15-fold higher than HIV+ population.
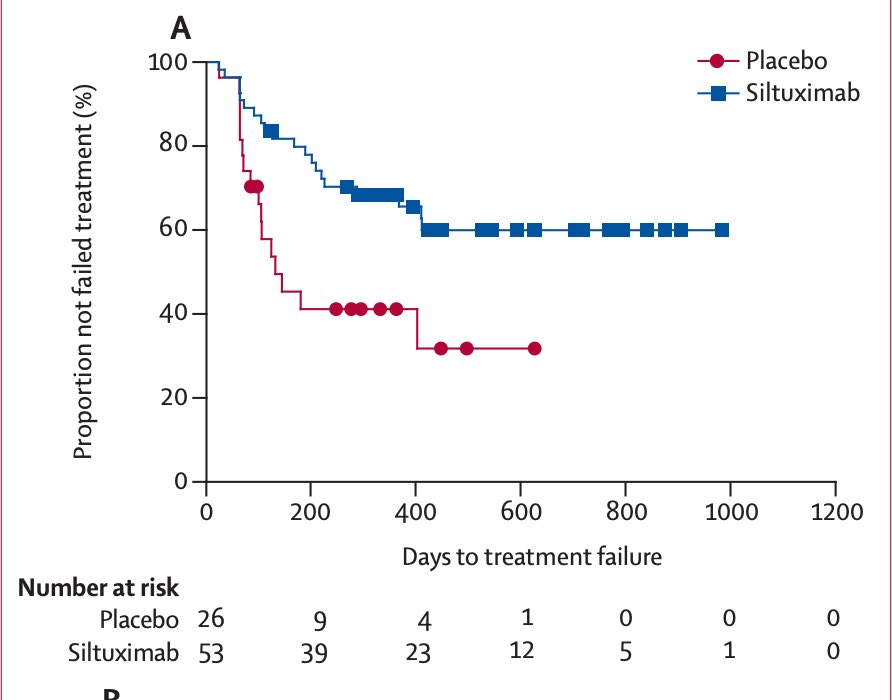
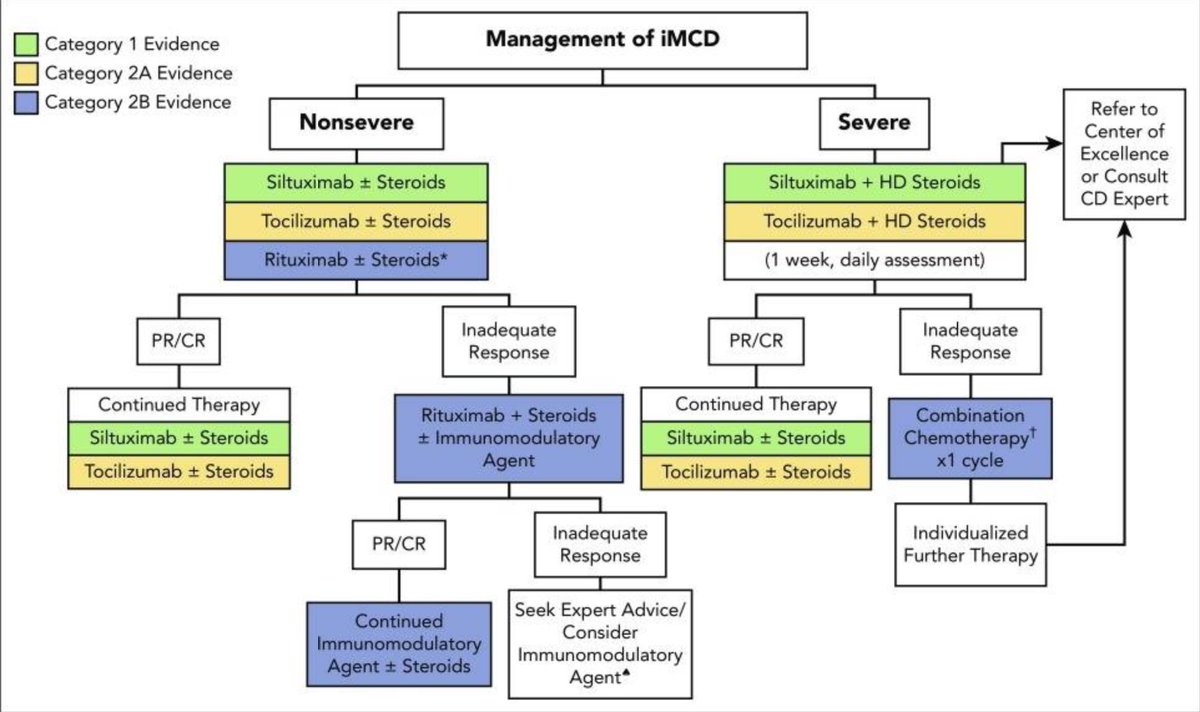
By 2014, siltuximab, a chimeric monoclonal antibody against IL-6, was approved by the @US_FDA