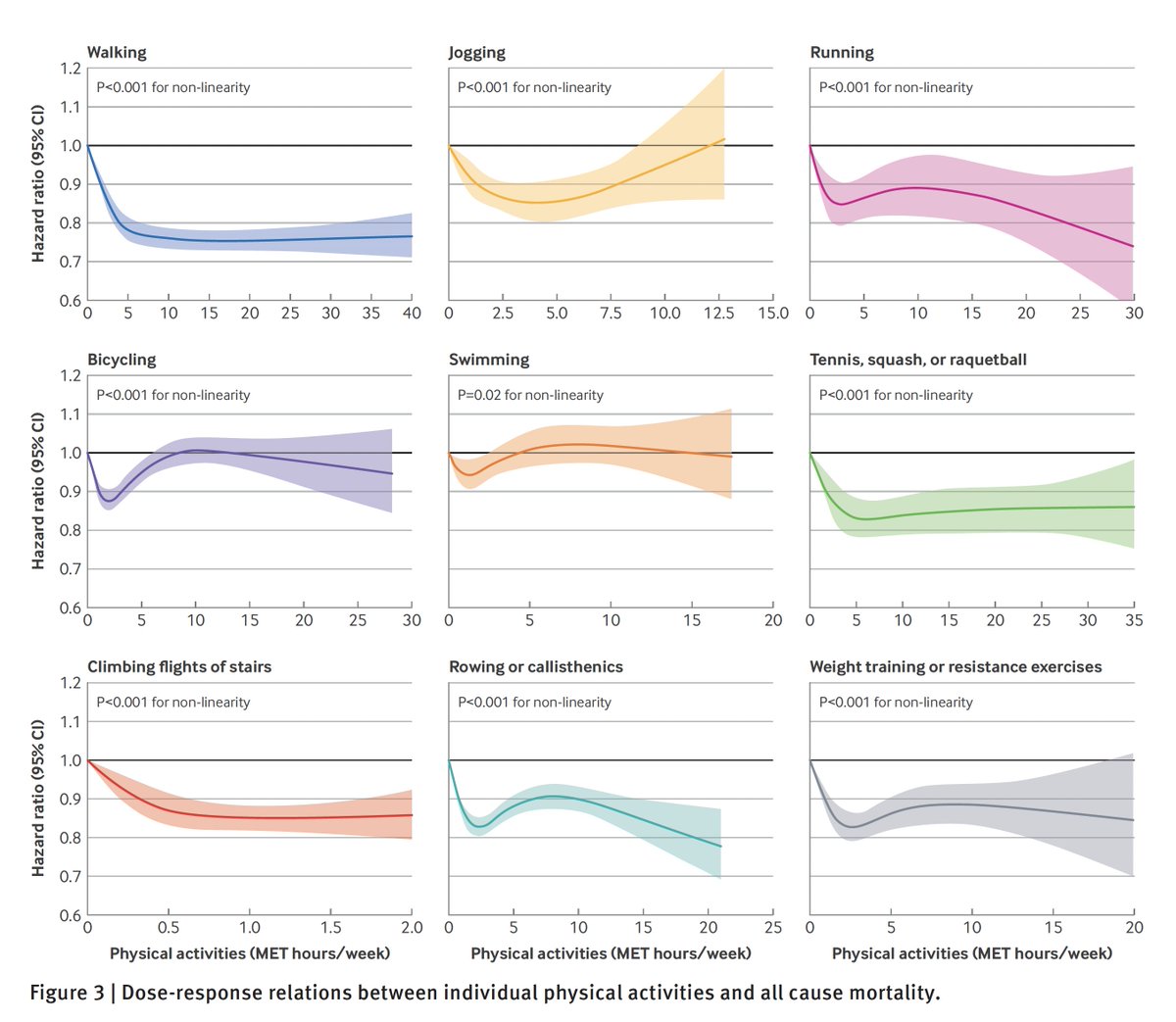
We have the protocols. Now we know how there will very likely be an Emergency Use Approval (EUA) for a vaccine prior to November 3. The company and political motivations are fully aligned.
1. The criteria for an EUA is that it "may be effective"
fda.gov/regulatory-inf…
1. The criteria for an EUA is that it "may be effective"
fda.gov/regulatory-inf…

2. Nearly every day we hear from @pfizer's CEO @AlbertBourla that they will know if their vaccine is working by the end of October.
Only the Data and Safety Monitoring Board is reviewing the data at specific intervals, the interim analyses
So how will they (Pfizer) know that?
Only the Data and Safety Monitoring Board is reviewing the data at specific intervals, the interim analyses
So how will they (Pfizer) know that?
3. The 1st interim analysis for that trial is at 32 events, infections, which can and likely will be mild. The stopping rules as reviewed by @biosbenk @EmoryRollins are "aggressive" and "unusual" for the number of interim analyses (4) and Bayesian approach
github.com/benkeser/pfize…
github.com/benkeser/pfize…
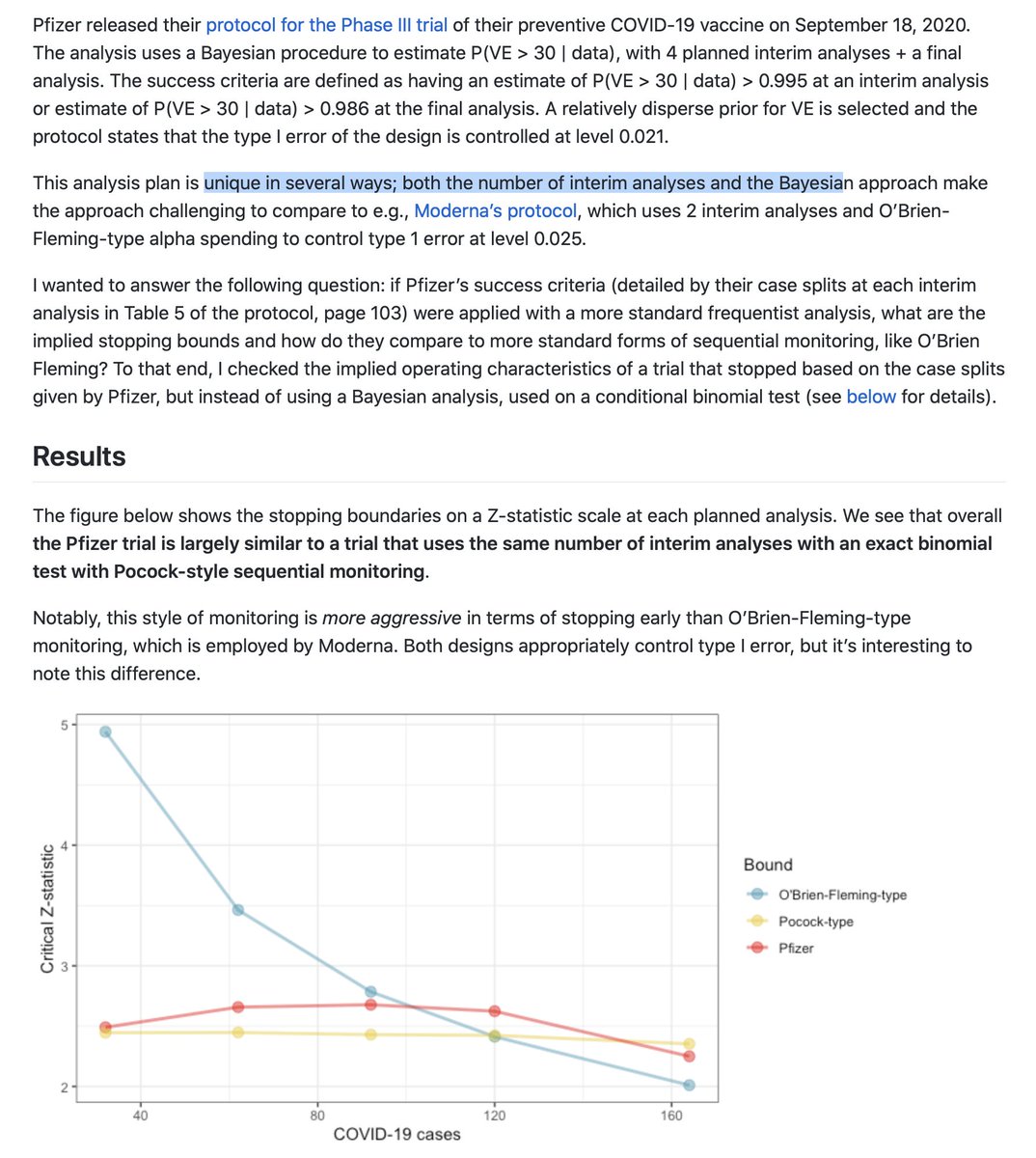
4. As presented in a poll, ≥ 26 infections in the placebo group at the 1st interim of 32 events would fulfill the stopping rule and support a claim of efficacy.
But the EUA only requires *"May be effective"* so even <26 placebo events could qualify
But the EUA only requires *"May be effective"* so even <26 placebo events could qualify
https://twitter.com/EricTopol/status/1306986697800908802
5. The DSMB reports to the sponsor. The trial data should not be unblinded unless it has been stopped (for futility, safety, or efficacy stopping rule) or completed. But it is easy to discern which group (vaccine or placebo) by the early adverse effects without unblinding.
6. Note the similarity in some symptoms for #COVID19 (which are the endpoint) and the early adverse effects of the vaccine (dose of 100 ug was used in Phase 3)
nejm.org/doi/full/10.10…
nejm.org/doi/full/10.10…
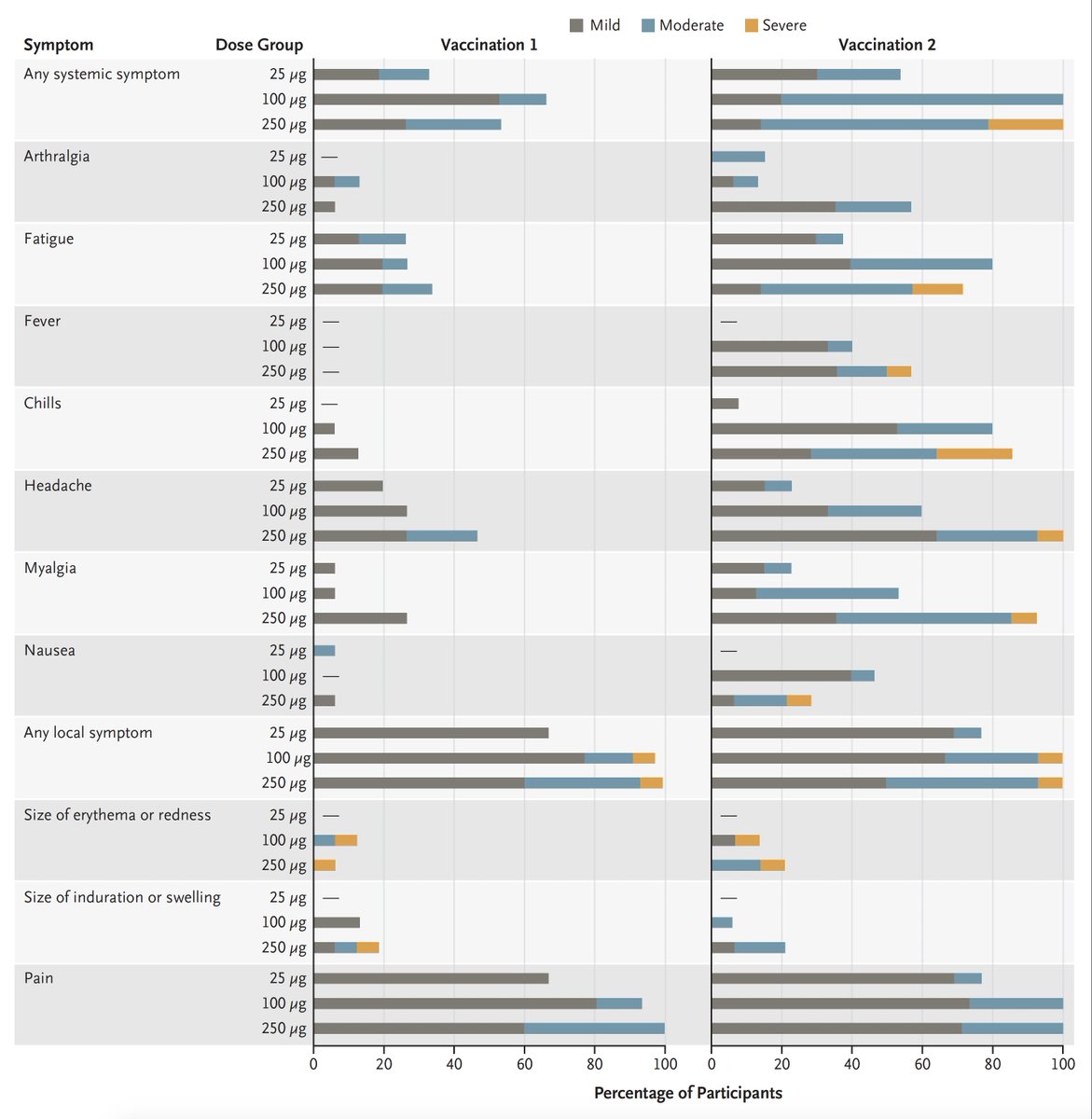
7. So with or without fulfilling pre-specified efficacy criteria at the 1st interim look, which will occur within weeks, Pfizer can apply for an EUA with the low threshold of "may be effective"
(If Pfizer hits the stopping rule and doesn't stop the trial, that's another story)
(If Pfizer hits the stopping rule and doesn't stop the trial, that's another story)
8. The EUA criteria are so minimal that even the Phase 1/2 serology data (NEJM paper above) could fulfill "may be effective" and that would equate to the Russian vaccine approval and roll out.
9. There has only been one FDA EUA for a vaccine in history, which was for anthrax, and that didn't go well theatlantic.com/health/archive… by @sarahzhang 

10. What if the FDA says NO, the data from 32 infections isn't enough? Clearly at that juncture there would be very limited data for the vaccine's safety.
The HHS can override that an issue the vaccine approval.
And we've already seen @HHSGov do that with FDA LDTs weeks ago.
The HHS can override that an issue the vaccine approval.
And we've already seen @HHSGov do that with FDA LDTs weeks ago.
11. The same issues apply for the @moderna_tx first interim analysis at 53 events, but a conventional O'Brien-Fleming stopping rule is being used and the 1° endpoint definition of infections is tighter
12. This is obviously a rush job—a race between companies with big political stakes. Once an EUA is issued for a vaccine, the whole landscape shifts. Safety issues could crop up later and engender mistrust. Ability to conduct placebo-controlled trials could be impaired.
12."Fast is Slow and Slow is Fast"
https://twitter.com/erictopol/status/1263898556600991745?lang=en
13. These are the most important clinical trials of our lifetime. There's no need for this rush. We need to do them right; get the Phase 3 trials completed as planned at 150-160 events. That will only require waiting weeks, it'll give us more confidence about efficacy and safety
14. Just as pressure was applied to @pfizer @BioNTech_Group and @moderna_tx to release their protocols, we need to apply intense pressure to the companies, the FDA, and HHS to preempt any EUA until Phase 3 trials are *fully* completed.
15. Translation, TLDR:
We need a shot in the light, not in the dark.
A matter of weeks to nail the efficacy issue down is well worth the wait.
Zero tolerance for company or governmental shortcuts and related back door BS.
We need a shot in the light, not in the dark.
A matter of weeks to nail the efficacy issue down is well worth the wait.
Zero tolerance for company or governmental shortcuts and related back door BS.

16. If there was any doubt about @HHSGov @SecAzar's plan to make sure there is an EUA for a vaccine before Nov 3 (see 10. above), then you can read this by @BySheilaKaplan
nytimes.com/2020/09/19/hea…
nytimes.com/2020/09/19/hea…
• • •
Missing some Tweet in this thread? You can try to
force a refresh