How can medical insurance companies NOT trick their customers!
Guidelines on handling of claims reported under Corona Virus: irdai.gov.in/ADMINCMS/cms/w…
(Images from thread below by @AnooBhu)



Guidelines on handling of claims reported under Corona Virus: irdai.gov.in/ADMINCMS/cms/w…
(Images from thread below by @AnooBhu)
https://twitter.com/AnooBhu/status/1309118055260323846



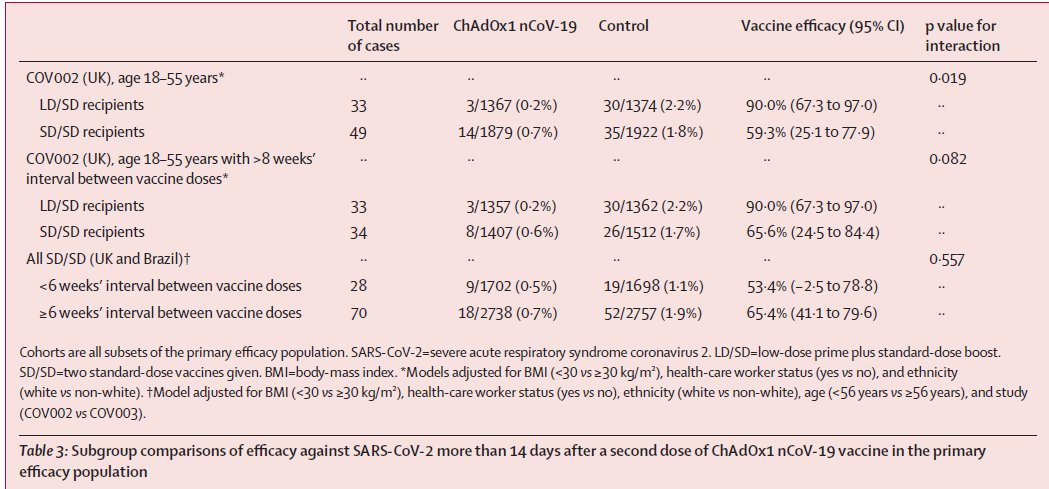
Ashraya Hospital, Chikmagalur, Karnataka
Bill splitting:
Doctor's visit charges alone is ~₹2 lakhs.
Separate hefty charges for COVID ICU, oxygen, NIV, etc.
Hospital admit: 24.08.20, discharge: 11.09.20
@malini_aisola @RemaNagarajan @inayat_s_kakar

Bill splitting:
Doctor's visit charges alone is ~₹2 lakhs.
Separate hefty charges for COVID ICU, oxygen, NIV, etc.
Hospital admit: 24.08.20, discharge: 11.09.20
@malini_aisola @RemaNagarajan @inayat_s_kakar
https://twitter.com/KirikKeerthi/status/1306815091283648512

Is @MaxHealthcare charging ₹ 8 lakhs when earlier estimated cost to the patient was ₹ 3.5 lakhs? What sort of inflation is this?
@malini_aisola @RemaNagarajan

@malini_aisola @RemaNagarajan
https://twitter.com/karan_arora18/status/1315155877482045440

❗️Action against 27 TN pvt hospitals for overcharging: 1 hospital pulled up for discharging a patient at midnight for being unable to pay bill, some others had to make settlements of up to Rs 2 lakh after charging excess fees.
newindianexpress.com/states/tamil-n…
https://twitter.com/Sinduj11/status/1316718769914347520
newindianexpress.com/states/tamil-n…

#COVID19 Pune: Measures to curtail overcharging ineffective.
The audit teams would only check bills of patients who've been charged more than ₹1.5 lakh, leaving majority of patients out of its purview. via @varshasuman
cc @malini_aisola @RemaNagarajan
thewire.in/health/covid-1…
The audit teams would only check bills of patients who've been charged more than ₹1.5 lakh, leaving majority of patients out of its purview. via @varshasuman
cc @malini_aisola @RemaNagarajan
thewire.in/health/covid-1…
Thread by @malini_aisola about billing of PPE & prices paid by patients for COVID treatment.



https://twitter.com/malini_aisola/status/1274181408991240192



Looks like weird refusal of mediclaim from @StarHealthIns.
Total bill is ₹ 6.41 lakh. Can one not use multiple insurance policies/claims to cover the bill?
@malini_aisola @RemaNagarajan @ramavenu @suchetadalal @MoneylifeIndia

Total bill is ₹ 6.41 lakh. Can one not use multiple insurance policies/claims to cover the bill?
@malini_aisola @RemaNagarajan @ramavenu @suchetadalal @MoneylifeIndia
https://twitter.com/Pratik15087653/status/1322483942612750336

@MaxHealthcare profiteering from COVID19 patients.
Also note that Tocilizumab is still mentioned when there's no +ve evidence and only -ve results from RCTs so far. Also, check how exaggerated other charges are on daily basis.
@malini_aisola


Also note that Tocilizumab is still mentioned when there's no +ve evidence and only -ve results from RCTs so far. Also, check how exaggerated other charges are on daily basis.
@malini_aisola
https://twitter.com/nikhil170824/status/1323618112814546944

Moolchand Healthcare @Moolchand_Hosp w/ post-COVID19 packages. Doctor consultation also for "Ayurveda".
Where is line between evidence-based treatment & quackery? Are patients clarified on method of treatment & evidences behind?
@malini_aisola

Where is line between evidence-based treatment & quackery? Are patients clarified on method of treatment & evidences behind?
@malini_aisola
https://twitter.com/das_seed/status/1323679889845792778

#MedicalHostage When patients or deceased are kept hostage by hospitals until bills are paid as per demand of hospital admins.
News story on issue covered by @SwetaDash93: thewire.in/health/private…
#HealthRacket

News story on issue covered by @SwetaDash93: thewire.in/health/private…
#HealthRacket
https://twitter.com/das_seed/status/1307661889279856641

1. Referral to fraudulent firm for loans by @MaxHealthcare staff and later not helping customer solve the issue arising due to it.
2. Overcharging of ambulance fees.

2. Overcharging of ambulance fees.
https://twitter.com/nikhil170824/status/1324827599978205184

Ex-servicepersons are also troubled by some ECHS Empanelled Hospitals. Some ECHS hospitals refuse to provide required medical attention which has also led to deaths.
@malini_aisola @RemaNagarajan @sanjg2k1 @drharshvardhan @MoHFW_INDIA @adgpi @PMOIndia


@malini_aisola @RemaNagarajan @sanjg2k1 @drharshvardhan @MoHFW_INDIA @adgpi @PMOIndia
https://twitter.com/satbirsm/status/1324260421587492864


Dhanwantri Nursing Home, MBD under Dr Ankit Verma took ₹137000 cash advance from patient. But doctor is refusing to give proper bill (w/ batch no.) for drugs on discharge. Patient's kin also concerned for genuineness of drugs prescribed/used. @ICMRDELHI


https://twitter.com/Adityaanandrock/status/1327148307337064448


Outright overcharging patient (e.g., multiple ICU charges for same day) by @NarayanaHealth.
Tocilizumab to 85 yr old lady w/ moderately severe or severe COVID19 seems medical negligence. There's only -ve evidences so far, see thread 👇.
@drlokeshksharma



Tocilizumab to 85 yr old lady w/ moderately severe or severe COVID19 seems medical negligence. There's only -ve evidences so far, see thread 👇.
@drlokeshksharma
https://twitter.com/das_seed/status/1294403398356414467



📢Cautionary notice for @Director_NABH accredited "allopathic" hospitals.
Some NABH accredited allopathic hospitals are employing AYUSH doctors for clinical duties @ ICU & other patient cares meant for MBBS RMOs & emergency doctors.
via @malini_aisola

Some NABH accredited allopathic hospitals are employing AYUSH doctors for clinical duties @ ICU & other patient cares meant for MBBS RMOs & emergency doctors.
via @malini_aisola
https://twitter.com/malini_aisola/status/1323675953583677440

Patient age 62 yrs, admitted on 28 Oct '20 @HospitalsApollo, Belapur. For 40 days of COVID19 related treatment, bill of ₹ 22 lakhs bill on 8 Dec '20. Family was told that further 1 month needed for recovery as per hospital, w/ est cost of ₹ 50 lakhs.

https://twitter.com/kunalsakhare22/status/1336353588491120645

Star Hospital, Hyderabad charging COVID19 patient for over ₹ 1 lakh per day. Also possibility of polypharmacy (incl. plasma therapy). Medicines/consumable alone are over ₹4 lakhs.
Are state govts & central govt monitoring COVID19 treatment costs?


Are state govts & central govt monitoring COVID19 treatment costs?
https://twitter.com/pnmsharma45/status/1382729030219100161


Details described by @RakeshMithai show thuggery of Medicare Hospital, Nagpur. It's plain loot & lack of empathy towards patients. They not only denied medical bill, but also claimed/bargained unnecessary amount for treatment patient didn't avail.



https://twitter.com/RakeshMithai/status/1381926999573233670



Farmers in Nashik being looted by some pvt hospitals: no billing, over charging, etc other than rude behavior towards patients & families. Also, Pvt hospitals seem to be taking benefit of lack of awareness about possible home care for mild COVID19.




https://twitter.com/PrakashBade5/status/1382315171108950023




CHL Hospital, Indore is overcharging for COVID19 treatment, & threat of no medicine if no payment.
8 days in hospital.
Consultancy: ₹26800
PPE kit: ₹9000
Oxygen: ₹24000
Room: ₹45400 (incl. of single day ICU)
....
Total: ~₹3.2 lakhs+ Service: ₹30000



8 days in hospital.
Consultancy: ₹26800
PPE kit: ₹9000
Oxygen: ₹24000
Room: ₹45400 (incl. of single day ICU)
....
Total: ~₹3.2 lakhs+ Service: ₹30000
https://twitter.com/Romil151/status/1376438064042532865



Ashoka Medicover Hospital Nashik charged ₹ 7.4 lakh to @j_baviskar's father huge bill for COVID19 treatment (15 Feb 2021 to 1 March 2021).
(Price of Ulinafic Inj seem to be more than online sale price.)
(Did they inject 7 Remdesivir &1 Tocilizumab too?)


(Price of Ulinafic Inj seem to be more than online sale price.)
(Did they inject 7 Remdesivir &1 Tocilizumab too?)
https://twitter.com/j_baviskar/status/1366289089587208192


• • •
Missing some Tweet in this thread? You can try to
force a refresh