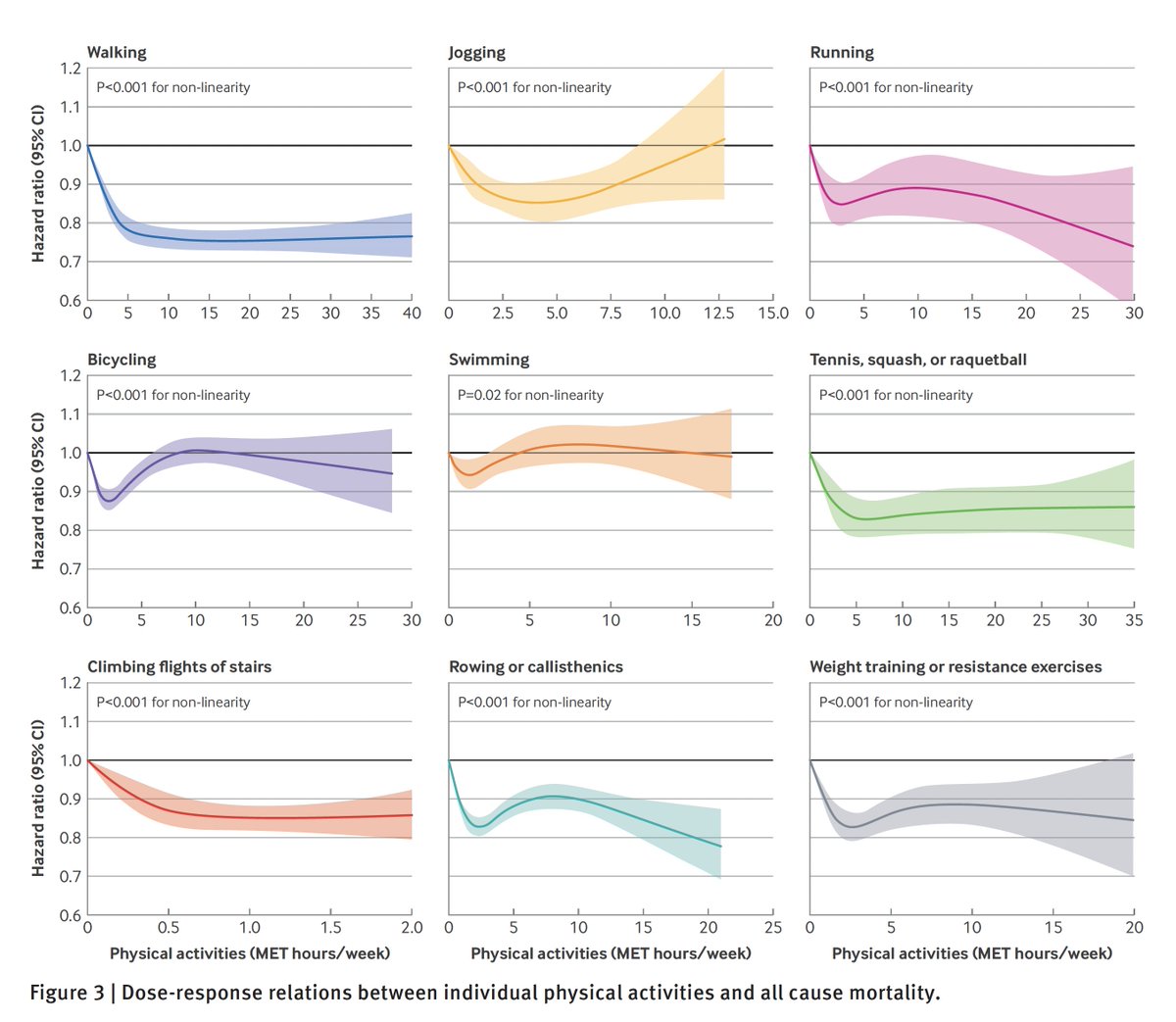
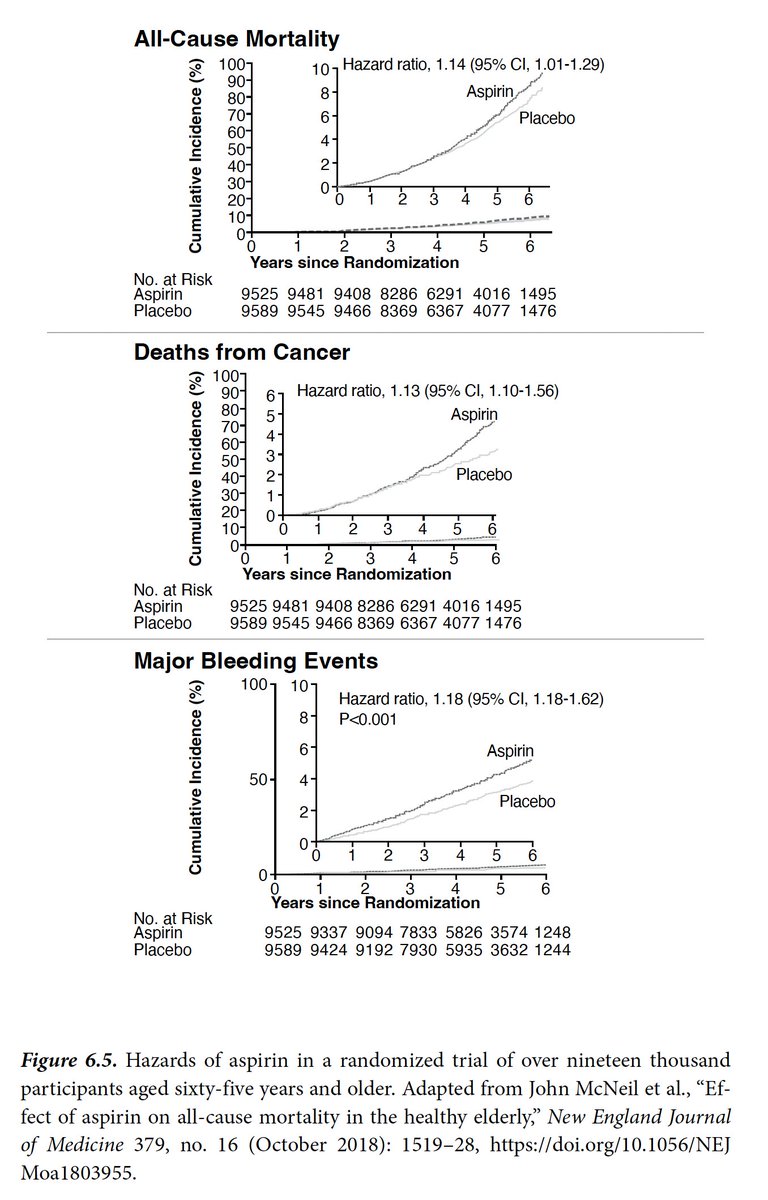
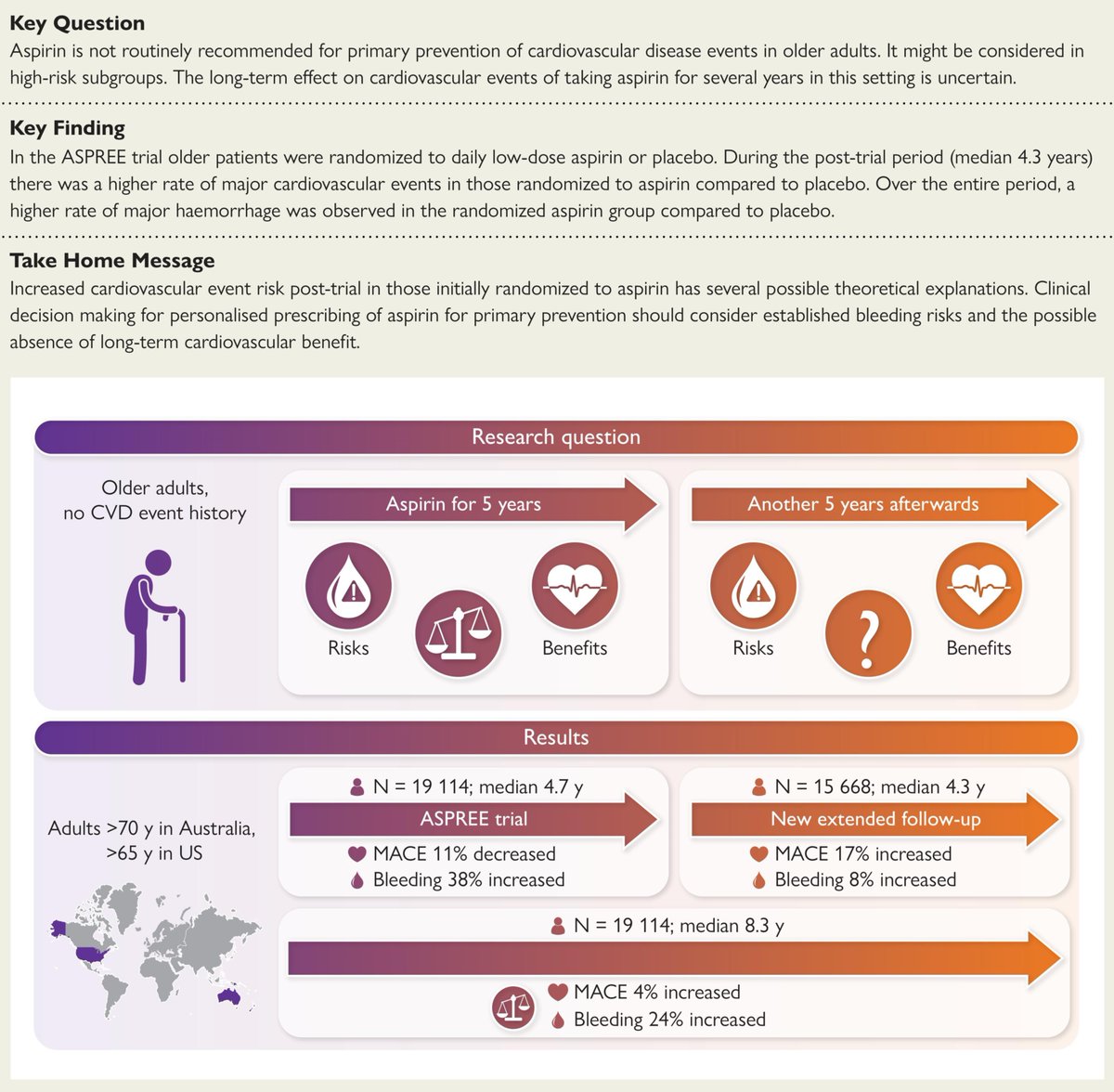
Why did we write a letter to a vaccine company about the need to complete its Phase 3 trial and provide compelling proof of its safety and efficacy?
Letter aboutblaw.com/TnB
An explanatory thread 1/
Letter aboutblaw.com/TnB
An explanatory thread 1/

Normally this process would be straightforward. But these are anything but normal times.
@Pfizer has its 1st interim analysis next week of 32 events (which include mild infections, such as a sore throat or headache +PCR virus). It has its own Data & Safety Monitoring Board. 2/
@Pfizer has its 1st interim analysis next week of 32 events (which include mild infections, such as a sore throat or headache +PCR virus). It has its own Data & Safety Monitoring Board. 2/
That DSMB has only 5 members. It could decide to stop the trial due to a predefined efficacy threshold (≥ 26 events w/placebo). It reports to Pfizer. The Pfizer CEO has said @FaceTheNation that its trial will have enough #COVID19 cases to apply for approval by late October. 3/
Or any of the 3 other interim analyses expected to be completed in October at 62, 92, 120 events before trial completion at 164 events.
At any point it could apply for an Emergency Use Authorization (EUA) to @US_FDA. 4/
At any point it could apply for an Emergency Use Authorization (EUA) to @US_FDA. 4/
The EUA criteria are summed up by 3 words:
"May Be Effective"
Almost anything would fit that rubric.
The FDA would normally adjudicate this decision.
But there is the rub. The @HHSgov @SecAzar, in conjunction w/ @WhiteHouse, has final authority on any EUA. That's the bypass 5/
"May Be Effective"
Almost anything would fit that rubric.
The FDA would normally adjudicate this decision.
But there is the rub. The @HHSgov @SecAzar, in conjunction w/ @WhiteHouse, has final authority on any EUA. That's the bypass 5/

This week, when the FDA announced it would tighten up the EUA criteria with longer safety surveillance and require some moderate-severe cases as events, @realDonaldTrump said he would not approve that
washingtonpost.com/politics/2020/…
@yabutaleb7 @lauriemcginley2 @jdawsey1 6/
washingtonpost.com/politics/2020/…
@yabutaleb7 @lauriemcginley2 @jdawsey1 6/
So the choke point here is that a company has to apply for an EUA. @realDonaldTrump @SecAzar cannot get a vaccine approved unless that happens. So our attention turns to Pfizer since it has been outspoken about its intent and timetable. 7/
But the other companies are into Phase 3 trials and @moderna_tx @AstraZeneca @JanssenUS @JNJNews may be in a position to apply for an EUA in the weeks ahead 8/ 

There's 1 DSMB for the other companies under @NIH, Operation Warp Speed (terrible name). OWS reports to HHS. Compared w/ @Pfizer's DSMB with 5 members, it has 10-15 members. The rosters or charters have not been disclosed, but this suggests better transdisciplinary expertise 9/
The DSMB's importance here cannot be overstated. Ideally it would include experts in clinical trials, biostats, bioethics, virology, immunology, vaccinology, and epidemiology. The DSMB is blinded to which arm (vaccine/placebo) in its review of safety and efficacy. 10/
We believe that the DSMB would share the concerns in our letter and want as strong an evidence base as possible, without holding up the vaccine approval, to support any vaccine's safety & efficacy. That would *exceed* the recent FDA EUA tightening of 2 months median follow-up 11/
Instead, we stipulated 2 months minimum. Since these trials include in their primary endpoint mild infections, the efficacy proof is lower than ideal (the 2° endpoint in the trials of moderate or severe illness would be far more compelling). 12/
So we are asking all the companies to fully complete their trials, analyze their data rigorously, and proceed with the knowledge that the stakes here could not be higher. It's our main exit strategy from the pandemic and we have to get it right 13/
That will help engender the public trust that is desperately needed. It will set the stage for high standards and success of multiple vaccine programs, which we also need. And the phenomenal science and velocity that got us to this point will not be put at risk. 14/f
Very good summary of the issue of trust in this recent piece by @maggiekb1 @FiveThirtyEight fivethirtyeight.com/features/how-t…
• • •
Missing some Tweet in this thread? You can try to
force a refresh