
@GemzME @sickanddamned I mean yes you will end up with rhabdomyolysis if you push too hard at the limits of dysfunctional mitochondria in muscle tissue.
I will also predict considerable myalgia in ME/CFS patients after little exercise. And yes, this appears to be the case.
I will also predict considerable myalgia in ME/CFS patients after little exercise. And yes, this appears to be the case.
@GemzME @sickanddamned Questions:
- What is the mechanism of indomethacin-induced mitochondrial fragmentation and ROS increase, and does it occur in a relevant immune response also?
- What is mechanism of COVID-19 myalgia? Is it related?
- What about myalgia in other viruses, esp w/ high lactate?
- What is the mechanism of indomethacin-induced mitochondrial fragmentation and ROS increase, and does it occur in a relevant immune response also?
- What is mechanism of COVID-19 myalgia? Is it related?
- What about myalgia in other viruses, esp w/ high lactate?
@GemzME @sickanddamned Indomethacin activates PKCζ-p38-DRP1 pathway, causing rapid mitochondrial fission and risking apoptosis.
jbc.org/content/early/…
Blocked by mitochondrial fission inhibitor mdivi-1
DRP1 is also induced by p53 in some viral infections, interestingly.
solvecfs.org/hhv-6-mediated…


jbc.org/content/early/…
Blocked by mitochondrial fission inhibitor mdivi-1
DRP1 is also induced by p53 in some viral infections, interestingly.
solvecfs.org/hhv-6-mediated…


@GemzME @sickanddamned HHV (above) and apparently also EBV (below) both *directly* induce DRP1 and cause mitochondria to fission.
nature.com/articles/s4139…
Meanwhile dengue does not.
A fish virus causing CNS necrosis also does it.
Reason: it prevents RLR-MAVS mediated interferon production!



nature.com/articles/s4139…
Meanwhile dengue does not.
A fish virus causing CNS necrosis also does it.
Reason: it prevents RLR-MAVS mediated interferon production!




@GemzME @sickanddamned These were just the first results pulled up: they happened to be for HHV-6 and EBV.
Let's see what CvB does.
pubmed.ncbi.nlm.nih.gov/28131843/
jvi.asm.org/content/jvi/91…
It also activates DRP1, apparently as a rather elaborate escape mechanism masking the virus from the immune system.

Let's see what CvB does.
pubmed.ncbi.nlm.nih.gov/28131843/
jvi.asm.org/content/jvi/91…
It also activates DRP1, apparently as a rather elaborate escape mechanism masking the virus from the immune system.


@GemzME @sickanddamned Meanwhile, according to the Prusty HHV-6 work, *pre-emptively* fragmenting mitochondria when warned by a nearby already-infected cell supposedly makes it more difficult for HHV-6 (and likely also other viruses) to infect cells, as @GemzME noted above.
google.com/amp/s/medicalx…


google.com/amp/s/medicalx…



@GemzME @sickanddamned Guess which other virus binds DRP1:
SARS-CoV, albeit in a manner similar to dengue:
ncbi.nlm.nih.gov/pmc/articles/P…
More generally, a number of viruses capable of infecting humans, especially persistently, seem to interact with DRP1.
frontiersin.org/articles/10.33…

SARS-CoV, albeit in a manner similar to dengue:
ncbi.nlm.nih.gov/pmc/articles/P…
More generally, a number of viruses capable of infecting humans, especially persistently, seem to interact with DRP1.
frontiersin.org/articles/10.33…


@GemzME @sickanddamned So then: what is in the plasma?
Warning signals from infected leukocytes, most likely!
Cells placed in plasma containing enough of these signals fragment their mitochondria pre-emptively, in an attempt to preserve their ability to use the MAVS pathway for interferon production.
Warning signals from infected leukocytes, most likely!
Cells placed in plasma containing enough of these signals fragment their mitochondria pre-emptively, in an attempt to preserve their ability to use the MAVS pathway for interferon production.
@GemzME @sickanddamned Links between Drp1 activation (mitochondrial fission) and inflammatory signaling in microglia (CNS macrophage equivalent):
grantome.com/grant/NIH/R21-…
The same upstream process causing Drp1 activation is probably also causing the observed elevation in CNS inflammatory markers.
grantome.com/grant/NIH/R21-…
The same upstream process causing Drp1 activation is probably also causing the observed elevation in CNS inflammatory markers.

@GemzME @sickanddamned Blocking Drp1 reduces neurotoxicity in a MPTP mouse Parkinsonism model:
nature.com/articles/ncomm…
Also in an α-synuclein Parkinsonism model:
actaneurocomms.biomedcentral.com/articles/10.11…
Drp1 activation involved in intestinal barrier damage caused by chronic kidney disease:
thno.org/v10p7384.pdf


nature.com/articles/ncomm…
Also in an α-synuclein Parkinsonism model:
actaneurocomms.biomedcentral.com/articles/10.11…
Drp1 activation involved in intestinal barrier damage caused by chronic kidney disease:
thno.org/v10p7384.pdf



@GemzME @sickanddamned Similarities in regional brain activity between ME/CFS and Parkinson's disease:
sciencedaily.com/releases/2014/…
CoQ10 depletion in both ME/CFS and Parkinsonism:
prohealth.com/library/coq10-…
This is consistent with what we would expect if both diseases partly involve Drp1 activation.


sciencedaily.com/releases/2014/…
CoQ10 depletion in both ME/CFS and Parkinsonism:
prohealth.com/library/coq10-…
This is consistent with what we would expect if both diseases partly involve Drp1 activation.



@GemzME @sickanddamned Anyway, the Prusty study looks conclusive for something in the plasma of ME/CFS patients, secreted by latently infected or perhaps previously-infected cells (not necessarily leukocytes), causing activation of Drp1 and ensuing mitochondrial fragmentation.
immunohorizons.org/content/4/4/201



immunohorizons.org/content/4/4/201




@GemzME @sickanddamned Likely something in the secretome of surviving infected cells.
There is also an antiviral effect, perhaps mediated by a different factor than the one activating Drp1.
It is not driven by IFN or TNF-α. In fact, ME/CFS IFN response is *worse*, as expected from MAVS disruption.


There is also an antiviral effect, perhaps mediated by a different factor than the one activating Drp1.
It is not driven by IFN or TNF-α. In fact, ME/CFS IFN response is *worse*, as expected from MAVS disruption.


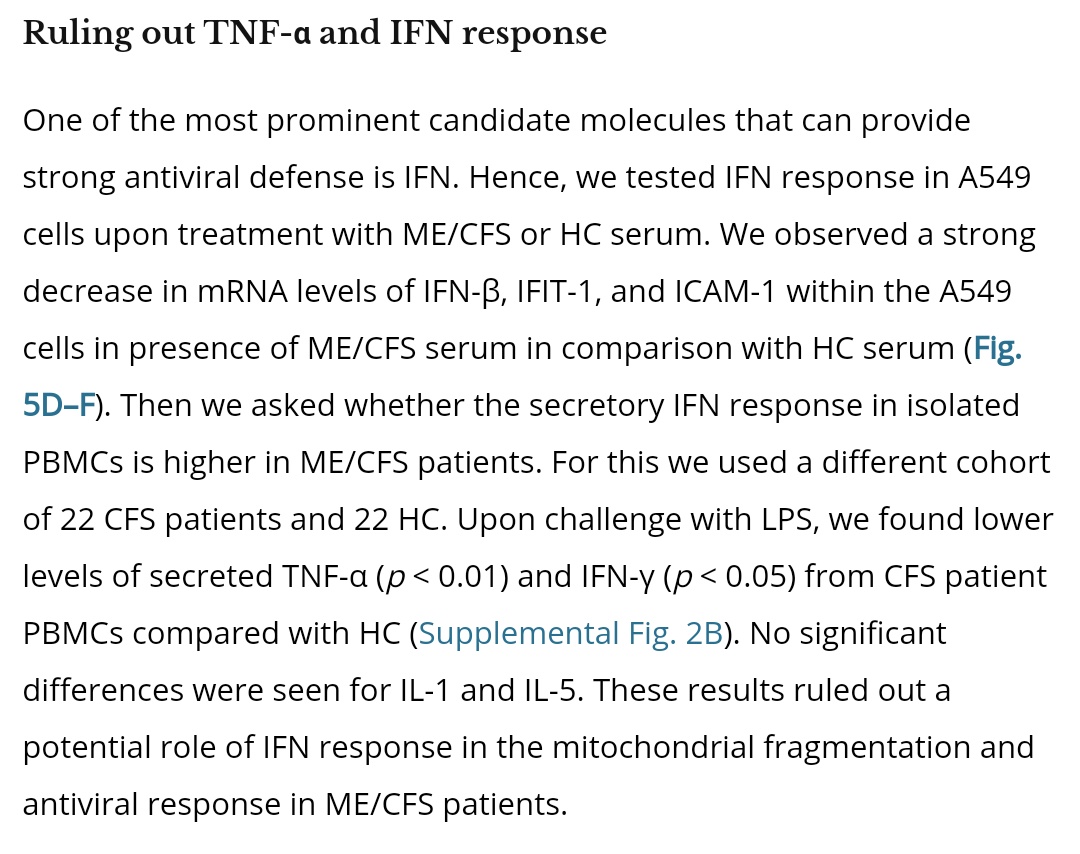
@GemzME @sickanddamned Here is an explanation for the pyruvate dehydrogenase issues observed above.
Note again the comment that only a small number of cells need to be latently infected with a suitable virus (or perhaps merely be survivors of a past infection) to secrete the relevant factors.



Note again the comment that only a small number of cells need to be latently infected with a suitable virus (or perhaps merely be survivors of a past infection) to secrete the relevant factors.



@GemzME @sickanddamned These claims match a different and very interesting paper I encountered several months ago, and to which I continue periodically returning, on the survival of cells infected with ordinarily lytic viruses and their inflammatory secretome:
@abledoc




@abledoc
https://twitter.com/__ice9/status/1308865401405747201?s=19


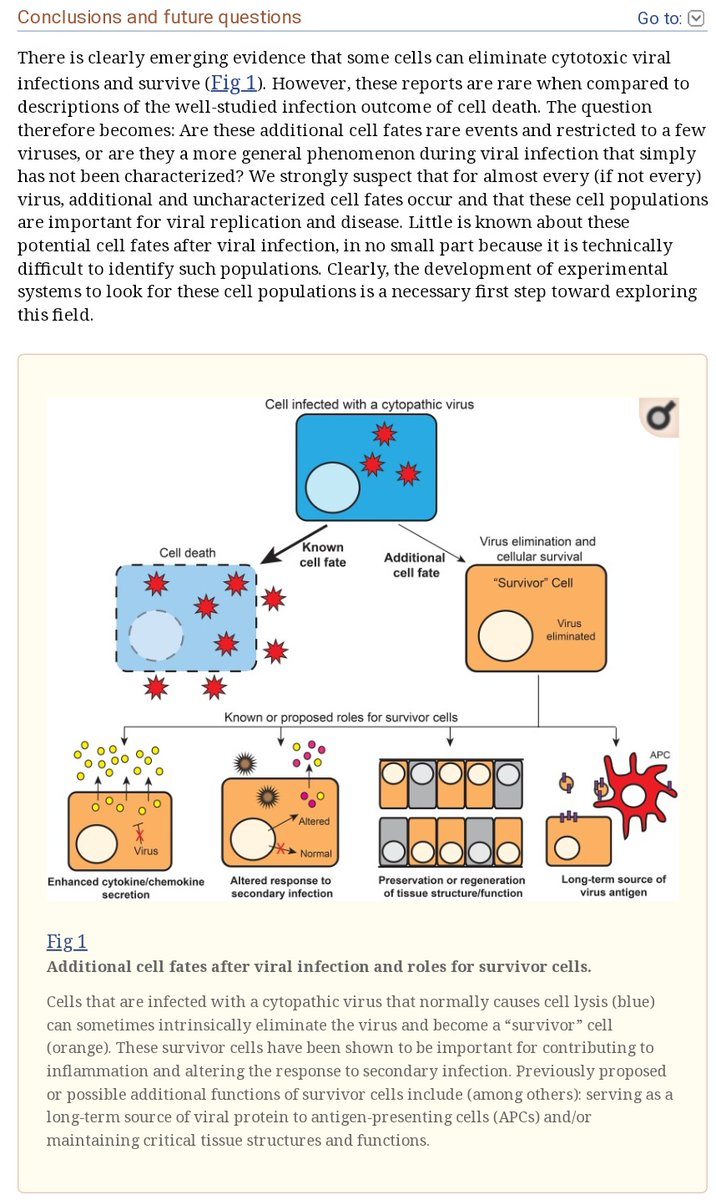

@GemzME @sickanddamned @abledoc I have also been recently informed of results strongly indicating the possibility of persistent infection (or at any rate persistent viral material) associated with a human coronavirus, a lytic and usually rapidly cleared virus:
In MS of all things.
https://twitter.com/microbeminded2/status/1315414098146926598?s=19
In MS of all things.
@GemzME @sickanddamned @abledoc These results indicate the possibility of a failure in some patients to fully clear all persistently, latently, or formerly infected cells following a given viral infection, leading to their survival and continued secretion of metabolically disruptive and/or inflammatory factors.
@GemzME @sickanddamned @abledoc Possible mechanisms:
NK cell impairments have been noted in ME/CFS and could limit clearance:
me-pedia.org/wiki/Natural_k…
Deficiencies in resolvin production could impair the cessation of inflammation:
jimmunol.org/content/196/8/…
T cell metabolism is impaired:
jci.org/articles/view/…



NK cell impairments have been noted in ME/CFS and could limit clearance:
me-pedia.org/wiki/Natural_k…
Deficiencies in resolvin production could impair the cessation of inflammation:
jimmunol.org/content/196/8/…
T cell metabolism is impaired:
jci.org/articles/view/…




@GemzME @sickanddamned @abledoc Mitochondrial fission might also not be the only virally associated alteration in mitochondrial function that could lead to increased ROS production and an early shift to glycolysis.
ncbi.nlm.nih.gov/pmc/articles/P…
Related results hint at reasons for diabetes as a COVID-19 risk factor.

ncbi.nlm.nih.gov/pmc/articles/P…
Related results hint at reasons for diabetes as a COVID-19 risk factor.


@GemzME @sickanddamned @abledoc The last one is particularly interesting given earlier speculation on analogies with the Warburg effect.
biorxiv.org/content/10.110…
Elevated lactate levels impair interferon signaling.
Impaired oxidative metabolism in relevant lymphocytes may also further impair clearance.

biorxiv.org/content/10.110…
Elevated lactate levels impair interferon signaling.
Impaired oxidative metabolism in relevant lymphocytes may also further impair clearance.


@GemzME @sickanddamned @abledoc Patients with post-viral ME/CFS may then be caught in a catch-22:
NK and T lymphocytes might be too metabolically constrained to clear lingering infected or formerly infected cells, and IFN signaling is likewise impaired.
Meanwhile those cells worsen the metabolic dysfunction.
NK and T lymphocytes might be too metabolically constrained to clear lingering infected or formerly infected cells, and IFN signaling is likewise impaired.
Meanwhile those cells worsen the metabolic dysfunction.
@GemzME @sickanddamned @abledoc Many viruses, notably including SARS-CoV-2, are also capable of forcing infected cells to degrade MHC and reduce its expression.
This creates substantial further difficulties for clearance of infected or formerly infected cells by cytotoxic lymphocytes.
biorxiv.org/content/10.110…
This creates substantial further difficulties for clearance of infected or formerly infected cells by cytotoxic lymphocytes.
biorxiv.org/content/10.110…

@GemzME @sickanddamned @abledoc Autoantibodies might also play some role in post-viral ME/CFS. Viral infections can cause autoimmune disorders.
me-pedia.org/wiki/Autoantib…
omf.ngo/2020/08/03/aut…
sciencedirect.com/science/articl…
However, the effect if so must be indirect:
ncbi.nlm.nih.gov/pmc/articles/P…



me-pedia.org/wiki/Autoantib…
omf.ngo/2020/08/03/aut…
sciencedirect.com/science/articl…
However, the effect if so must be indirect:
ncbi.nlm.nih.gov/pmc/articles/P…




@GemzME @sickanddamned @abledoc It is not clear that autoimmune phenomena of this nature would cause the core mitochondrial symptoms in ME/CFS.
They may be more associated with POTS and/or dysautonomia in general, given binding profiles indicated above, and with connective tissue or neurological inflammation.
They may be more associated with POTS and/or dysautonomia in general, given binding profiles indicated above, and with connective tissue or neurological inflammation.
@GemzME @sickanddamned @abledoc Just from my perspective, it appears POTS/dysautonomia and persistent post-viral joint or nerve inflammation would be better viewed as common comorbidities with ME/CFS than aspects of it per se. Mechanisms seem too different, and all three could be induced by viral infection.
@GemzME @sickanddamned @abledoc That would raise the slightly nuanced question of how best to treat a comorbid failure to clear lingering viral material and an autoimmune condition. Need more data on POTS in particular.
In any event, clinical trials need to rigorously distinguish between these patient groups.
In any event, clinical trials need to rigorously distinguish between these patient groups.
@GemzME @sickanddamned @abledoc Further context:
me-pedia.org/wiki/Postural_…
Adrenergic and perhaps muscarinic receptor involvement appears overwhelmingly likely to be involved in POTS.
me-pedia.org/wiki/Postural_…
Adrenergic and perhaps muscarinic receptor involvement appears overwhelmingly likely to be involved in POTS.
@GemzME @sickanddamned @abledoc POTS appears to be caused by an underlying neurological problem in most cases, and viewing it as a form of dysautonomia is likely correct.
On the bright side, symptoms are usually fairly treatable.
https://twitter.com/__ice9/status/1319395758064472064?s=19
On the bright side, symptoms are usually fairly treatable.
@GemzME @sickanddamned @abledoc Back to the subject of ME/CFS:
The lipid paper above is also consistent with the idea that there may be a persistent or latent viral infection in many ME/CFS cases.
Compare:
Plasma sphingolipids up:
nature.com/articles/s4159…
Choline demand up:
frontiersin.org/articles/10.33…

The lipid paper above is also consistent with the idea that there may be a persistent or latent viral infection in many ME/CFS cases.
Compare:
Plasma sphingolipids up:
nature.com/articles/s4159…
Choline demand up:
frontiersin.org/articles/10.33…


@GemzME @sickanddamned @abledoc Elevated lactate dehydrogenase levels in ME/CFS are also suspicious, given association with viral infection and tissue damage.
medlineplus.gov/lab-tests/lact…
medrxiv.org/content/10.110…
me-pedia.org/wiki/Lactate_d…
healthrising.org/blog/2019/12/2…
Circumstantial, but points in the same direction.



medlineplus.gov/lab-tests/lact…
medrxiv.org/content/10.110…
me-pedia.org/wiki/Lactate_d…
healthrising.org/blog/2019/12/2…
Circumstantial, but points in the same direction.




@GemzME @sickanddamned @abledoc Persistent viral infection can cause vitamin B6 (pyridoxine etc.) deficiency:
ijnpnd.com/article.asp?is…
Vitamin B6 used for antiviral immune response (impaired by deficiency):
pubmed.ncbi.nlm.nih.gov/8302491/
ncbi.nlm.nih.gov/pmc/articles/P…
ME/CFS shows B6 deficiency:
ncbi.nlm.nih.gov/pmc/articles/P…



ijnpnd.com/article.asp?is…
Vitamin B6 used for antiviral immune response (impaired by deficiency):
pubmed.ncbi.nlm.nih.gov/8302491/
ncbi.nlm.nih.gov/pmc/articles/P…
ME/CFS shows B6 deficiency:
ncbi.nlm.nih.gov/pmc/articles/P…




@GemzME @sickanddamned @abledoc Article on "post-viral fatigue syndrome," an earlier term for what seems to be essentially ME/CFS when strongly suspected to be triggered by a viral infection:
me-pedia.org/wiki/Postviral…
Causes are almost all viruses known to persist in humans, including SARS-CoV.



me-pedia.org/wiki/Postviral…
Causes are almost all viruses known to persist in humans, including SARS-CoV.




@GemzME @sickanddamned @abledoc SARS-CoV, SARS-CoV-2, and some HCoVs all show indications of potentially persistent infection in various tissues.
wwwnc.cdc.gov/eid/article/26…
jvi.asm.org/content/82/11/…
tandfonline.com/doi/full/10.10…
thelancet.com/journals/ebiom…




wwwnc.cdc.gov/eid/article/26…
jvi.asm.org/content/82/11/…
tandfonline.com/doi/full/10.10…
thelancet.com/journals/ebiom…
https://twitter.com/__ice9/status/1319333789978578944?s=19




@GemzME @sickanddamned @abledoc In that sense, COVID-19-associated ME/CFS, which appears to be only a particular specific subset of reported COVID-19 post-acute sequelae, might *literally* involve a 'long' duration persistent infection rather than merely aftereffects like the others.
https://twitter.com/__ice9/status/1319509433601372166?s=19
@GemzME @sickanddamned @abledoc Rarely, SARS-CoV-2 has been reported to *reactivate* months after acute recovery and seroconversion, e.g. after potent immunosuppressants.
jhoonline.biomedcentral.com/articles/10.11…
Many relapses are of course also known:
frontiersin.org/articles/10.33…
ncbi.nlm.nih.gov/pmc/articles/P…
health.economictimes.indiatimes.com/news/diagnosti…



jhoonline.biomedcentral.com/articles/10.11…
Many relapses are of course also known:
frontiersin.org/articles/10.33…
ncbi.nlm.nih.gov/pmc/articles/P…
health.economictimes.indiatimes.com/news/diagnosti…




@GemzME @sickanddamned @abledoc Back on the subject of ME/CFS and chronic infection:
Patients with SLE, RA, and especially PsA have low thymosin-α levels:
ncbi.nlm.nih.gov/pmc/articles/P…
It is also lower in patients with chronic infections:
tandfonline.com/doi/full/10.10…
But not acute:
pubmed.ncbi.nlm.nih.gov/2572736/


Patients with SLE, RA, and especially PsA have low thymosin-α levels:
ncbi.nlm.nih.gov/pmc/articles/P…
It is also lower in patients with chronic infections:
tandfonline.com/doi/full/10.10…
But not acute:
pubmed.ncbi.nlm.nih.gov/2572736/



@GemzME @sickanddamned @abledoc Thymosin-α1 was superior to interferon α in clearing hepatitis B infection, and produced decent results in several other trials:
ncbi.nlm.nih.gov/pmc/articles/P…
ncbi.nlm.nih.gov/books/NBK75853/
This was before widespread use of modern nucleoside analogues.
I see no data for ME/CFS, though.

ncbi.nlm.nih.gov/pmc/articles/P…
ncbi.nlm.nih.gov/books/NBK75853/
This was before widespread use of modern nucleoside analogues.
I see no data for ME/CFS, though.


@GemzME @sickanddamned @abledoc Community results for thymosin α1 are favorable, along with thymosin β4 and supposedly thymulin, and it seems worth watching for further research findings.
However, I think direct evidence is limited and these are mostly being tried for putative mechanism reasons.
However, I think direct evidence is limited and these are mostly being tried for putative mechanism reasons.
@GemzME @sickanddamned @abledoc The dsRNA-based selective TLR-3 agonist (important for inflammatory reasons) rintatolimod has been successful in several placebo-controlled DBRCTs and multiple open-level trials for ME/CFS.
pubmed.ncbi.nlm.nih.gov/27045557/
Paper also notes likely heterogeneity of triggering infections.



pubmed.ncbi.nlm.nih.gov/27045557/
Paper also notes likely heterogeneity of triggering infections.




@GemzME @sickanddamned @abledoc More on rintatolimod (Ampligen):
me-pedia.org/wiki/Ampligen
IV only, seemingly.
The FDA is still getting in the way. It is approved for early access in the EU, Turkey, and Argentina.
History dates to the 1960s, with clinical efficacy in ME/CFS shown as early as the mid-1990s.



me-pedia.org/wiki/Ampligen
IV only, seemingly.
The FDA is still getting in the way. It is approved for early access in the EU, Turkey, and Argentina.
History dates to the 1960s, with clinical efficacy in ME/CFS shown as early as the mid-1990s.




@GemzME @sickanddamned @abledoc Ampligen also works well as an adjuvant for some experimental intranasal vaccines:
ncbi.nlm.nih.gov/pmc/articles/P…
I have not found any evidence for it being tested by subcutaneous or intramuscular injection instead of IV.
Other TLR agonists are, though.
ncbi.nlm.nih.gov/pmc/articles/P…

ncbi.nlm.nih.gov/pmc/articles/P…
I have not found any evidence for it being tested by subcutaneous or intramuscular injection instead of IV.
Other TLR agonists are, though.
ncbi.nlm.nih.gov/pmc/articles/P…


@GemzME @sickanddamned @abledoc Unfortunately, the TLR3 pathway is independent of PKR rather than containing it, so e.g. indomethacin (off-target unconditional PKR activator) likely would not do the same thing.
jimmunol.org/content/181/4/…
Downstream targets differ fairly broadly.



jimmunol.org/content/181/4/…
Downstream targets differ fairly broadly.




@GemzME @sickanddamned @abledoc For specific pathogens, relevant post-entry antivirals sometimes seem to work.
For instance, long-term treatment with high doses of herpes antivirals was moderately successful in several ME/CFS studies:
phoenixrising.me/treating-cfs-c…
ncbi.nlm.nih.gov/pmc/articles/P…


For instance, long-term treatment with high doses of herpes antivirals was moderately successful in several ME/CFS studies:
phoenixrising.me/treating-cfs-c…
ncbi.nlm.nih.gov/pmc/articles/P…



@GemzME @sickanddamned @abledoc If this worked, then long-term treatment with appropriate post-entry antivirals or innate immunostimulants against SARS-CoV-2 may do something useful for persistent COVID-19 cases, including those with severe fatigue.
See here for some possible options:
See here for some possible options:
https://twitter.com/__ice9/status/1319378944303431680?s=19
@GemzME @sickanddamned @abledoc Anecdotally, there have been reports of treating persistent COVID-19 symptoms with post-entry antivirals, e.g.:
github.com/symmetry-covid…
Granted, this is N=1 and does not appear to have been an ME/CFS-like situation per se. But it does suggest value in persistent infection.
github.com/symmetry-covid…
Granted, this is N=1 and does not appear to have been an ME/CFS-like situation per se. But it does suggest value in persistent infection.
@GemzME @sickanddamned @abledoc This reminded me to look for B6 evidence, given results above on low B6 levels in ME/CFS. I found no trials, but I did see favorable community reports:
ifsblog.wordpress.com/2017/05/03/vit…
Used for sphingolipids:
forums.phoenixrising.me/threads/what-i…
Related to low FAD:
pnas.org/content/113/37…


ifsblog.wordpress.com/2017/05/03/vit…
Used for sphingolipids:
forums.phoenixrising.me/threads/what-i…
Related to low FAD:
pnas.org/content/113/37…



@GemzME @sickanddamned @abledoc Importantly, high dose vitamin B6 supplements should use P-5-P rather than pyridoxine, as the latter has neurotoxic properties:
https://twitter.com/__ice9/status/1206055014210387974?s=19
@GemzME @sickanddamned @abledoc Pondering indomethacin's PLR activity again, I went looking for small-molecule TLR agonists.
I did not find any for TLR3 -- only TLR7 (also handles RNA viruses, but triggers IRF7, not IRF3).
The only approved drug listed here is imiquimod, not viable.
academic.oup.com/jac/article/67…



I did not find any for TLR3 -- only TLR7 (also handles RNA viruses, but triggers IRF7, not IRF3).
The only approved drug listed here is imiquimod, not viable.
academic.oup.com/jac/article/67…




@GemzME @sickanddamned @abledoc Imiquimod is not safe for systemic use.
It has useful properties against warts, skin cancer, and perhaps even superficial sarcomas, but cannot be used against deeper viral infections.



It has useful properties against warts, skin cancer, and perhaps even superficial sarcomas, but cannot be used against deeper viral infections.




@GemzME @sickanddamned @abledoc Also worth checking screens for IRF3 or its own targets, bypassing TLR3 and dropping NF-κB.
RIPA activators kill infected cells by apoptosis:
- doxorubicin
- pyrvinium pamoate
ncbi.nlm.nih.gov/pmc/articles/P…
Doxorubicin is impractical, but pyrvinium works at hittable concentrations.



RIPA activators kill infected cells by apoptosis:
- doxorubicin
- pyrvinium pamoate
ncbi.nlm.nih.gov/pmc/articles/P…
Doxorubicin is impractical, but pyrvinium works at hittable concentrations.




@GemzME @sickanddamned @abledoc Full results excluding topicals, antipsychotics, potent sedatives, chemo drugs, GI-only, steroids:
- pyrvinium
- loperamide
- nicergoline
- acemetacin
- proguanil
- methotrexate
- cyproheptadine
- pimethixene
- labetalol
- vinpocetine
- chloroxine
- celecoxib
- isoconazole

- pyrvinium
- loperamide
- nicergoline
- acemetacin
- proguanil
- methotrexate
- cyproheptadine
- pimethixene
- labetalol
- vinpocetine
- chloroxine
- celecoxib
- isoconazole
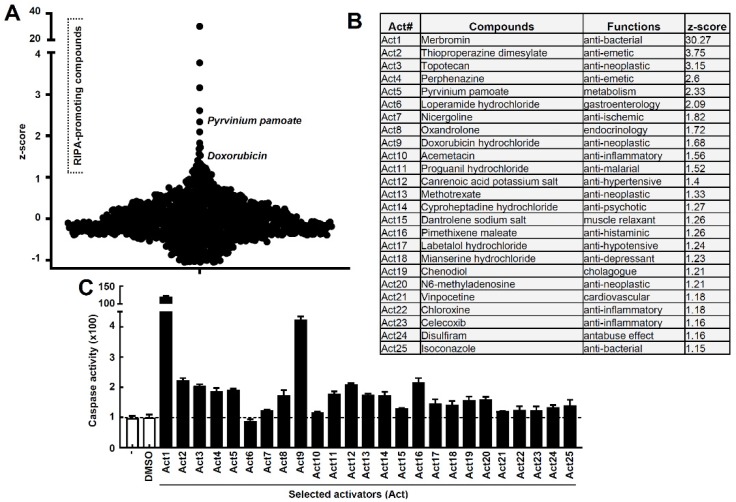

@GemzME @sickanddamned @abledoc Methotrexate has significant adverse effects and practitioners report poor tolerability in ME/CFS:
healthrising.org/blog/2017/02/1…
Loperamide has substantial off-target effects. Similar CoV antiviral concentrations are unhittable.
Typo above, "PKR" not "PLR" for indomethacin.
healthrising.org/blog/2017/02/1…
Loperamide has substantial off-target effects. Similar CoV antiviral concentrations are unhittable.
Typo above, "PKR" not "PLR" for indomethacin.
@GemzME @sickanddamned @abledoc Nicergoline: ergot derivative (supposedly the safe one); I see no antiviral data.
Acemetacin: worth investigating.
Atovaquone-proguanil: hittable antiviral vs. SARS-CoV-2.
Cyproheptadine: has antiviral effects, need more data.
Pimethixene: fairly potent.
Others look weaker.
Acemetacin: worth investigating.
Atovaquone-proguanil: hittable antiviral vs. SARS-CoV-2.
Cyproheptadine: has antiviral effects, need more data.
Pimethixene: fairly potent.
Others look weaker.
@GemzME @sickanddamned @abledoc Acemetacin is an indomethacin prodrug, so indomethacin triggers RIPA after all:
elsevier.es/en-revista-ann…
Atovaquone inhibits SARS-CoV-2 at hittable EC90:
biorxiv.org/content/10.110…
Perhaps by nucleotide depletion:
ncbi.nlm.nih.gov/pmc/articles/P…
No reports for proguanil. May still be real.
elsevier.es/en-revista-ann…
Atovaquone inhibits SARS-CoV-2 at hittable EC90:
biorxiv.org/content/10.110…
Perhaps by nucleotide depletion:
ncbi.nlm.nih.gov/pmc/articles/P…
No reports for proguanil. May still be real.
@GemzME @sickanddamned @abledoc Pimethixene is actually very similar to cyproheptadine and may be a stronger sedative. Only available in France, Brazil, Tunisia.
Cyproheptadine is an HCV entry inhibitor. RIPA effects look a bit weak too.
jvi.asm.org/content/91/2/e…
I see no antiviral results for nicergoline.
Cyproheptadine is an HCV entry inhibitor. RIPA effects look a bit weak too.
jvi.asm.org/content/91/2/e…
I see no antiviral results for nicergoline.

@GemzME @sickanddamned @abledoc Unfortunately, pyrvinium pamoate has little to no oral bioavailability. EC50 for related CoVs was marginal as-is, so this is likely not a practical option either.
@GemzME @sickanddamned @abledoc That leaves the following approved oral TLR3-bypassing IRF3-mediated RIPA activators inhibiting SARS-CoV-2 or related CoV replication at hittable EC50 or better:
- indomethacin, or prodrug acemetacin
- atovaquone-proguanil
I predict both as useful in persistent COVID-19.
- indomethacin, or prodrug acemetacin
- atovaquone-proguanil
I predict both as useful in persistent COVID-19.
@GemzME @sickanddamned @abledoc Unfortunately, this isn't really new. Indomethacin was already suspected because of its PKR activity. Still, more evidence.
Probably the main contribution from this paper was finding a use for proguanil, given atovaquone itself already looks hittable.
https://twitter.com/__ice9/status/1319376785419755520?s=19
Probably the main contribution from this paper was finding a use for proguanil, given atovaquone itself already looks hittable.
• • •
Missing some Tweet in this thread? You can try to
force a refresh











