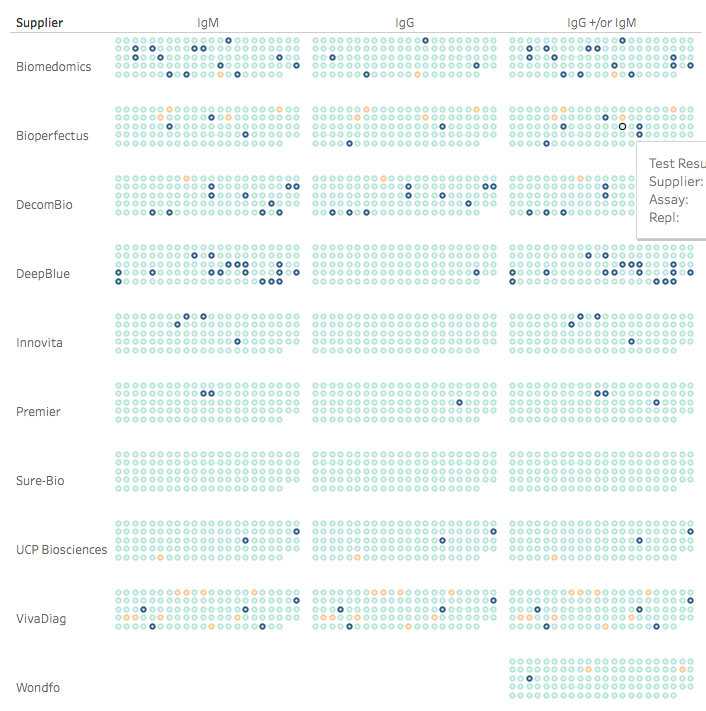
Here's a Christmas gift: Turkey says Sinovac's COVID vaccine is 91.25% effective. That's a prelim result based on <30 cases but it adds to an earlier announcement from Brazil of >50% efficacy.
This vaccine is an inactivated virus. That's interesting...
reut.rs/37MIOIr
This vaccine is an inactivated virus. That's interesting...
reut.rs/37MIOIr
Inactivated virus (aka whole-killed virus or WKV) is the oldest recipe in the vaccine cookbook. The 1st polio (Salk) and flu vaccines work this way. You kill the virus with chemicals, mix with adjuvant, and inject. APCs process and present viral antigens to activate B cells.
In early versions of my #coronadeck I suggested the CDC organize the production of a WKV vacccine. It's very simple and requires no new technology. So why did we not do it? There was a scientific reason but also economic/political reasons.
The scientific reason is fear of antibody-mediated enhancement. This famously happened with an inactivated RSV vaccine in the 1970s. This vaccine produced low-affinity antibodies (believed to be due to chemical modification of the target antigens).
The low-affinity antibodies ended up making disease worse, perhaps by facilitating viral entry into cells instead of blocking it, in a process called antibody-mediated enhancement (AME). Vaccinated children died from RSV at higher rates than unvaccinated children. Oops.
Possible AME in COVID19 got a lot of attention early on. It was a concern also raised for the SARSCoV2 monoclonal antibodies, so experiments were done to rule out mAb-mediated virus uptake. The mAbs ended up safe and efficacious and are now available for all high-risk patients.
Given RSV experience, the case was made to choose a vaccine approach that could reduce AME risk. The mRNA vaccines produce viral proteins without chemical modification. Pfizer/BioNTech's for example is simply full-length SARSCOV2.
https://twitter.com/markwbudde/status/1341430999381495811
The Moderna vaccine is actually a fragment of S protein in a "prefusion-stabilized conformation". They make a big deal about the design comes out of previous research (below) but the Pfizer/BioNTech results show that the designed aspects were unnecessary.
nature.com/articles/s4158…
nature.com/articles/s4158…
Now which approach, WKV or mRNA, actually had the better scientific premise for application to SARSCoV2? Logic and experience say when given just one shot against a new target, you should choose the weapon that worked in the most similar past situation. For SARSCoV2 that was WKV.
mRNA vaccines had been shown to work for MERS in mice, but MERSCoV2 is only 50% identical to SARSCoV2. Also mouse results are of limited generalization to humans; mice are quite different from humans in pathogenesis and immunity.
genengnews.com/news/mers-vacc…
genengnews.com/news/mers-vacc…
mRNA vaccines had been tested in humans for flu and rabies, but not for any coronaviruses. Thus, to play the typical grant reviewer, we could say applicants lack preliminary evidence that mRNA expressing a coronavirus S protein would produce robust immune responses in humans.
In contrast, WKV vaccines for SARSCoV, 80% identical to SARSCoV2, had been tested in humans and shown to be safe and to produce neutralizing antibodies more than a decade ago. Similar data were only obtained with mRNA vaccines this summer (for SARSCoV2).
pubmed.ncbi.nlm.nih.gov/19538115/
pubmed.ncbi.nlm.nih.gov/19538115/
The antibodies produced in humans after WKV inoculation could neutralize virus in vitro, which suggests that AME would not be a problem. This was SARSCoV, not SARSCoV2, but the S proteins are 76% identical, so there was a good chance a SARSCoV2 WKV vaccine would work too.
In addition, WKV vaccines, because they are fixed, are not perishable. Thus, they don't need to be kept frozen using dry ice or freezers.
So why did Europe and the US both choose mRNA vaccines (never before tested for coronaviruses in humans) and not WKV (proven efficacy for antibody production against coronavirus in humans)?
You could worry that SARSCoV2 WKV could cause AME when SARSCoV1 WKV didn't due to the 20% sequence difference, but you could also worry that a mRNA vaccine could cause AME by not stimulating APCs long enough for affinity maturation to create high-affinity antibodies.
I think one reason why Western countries pursued mRNA vaccines for COVID19, and not WKV vaccines despite WKV requiring no new technology, was precisely because WKV *didn't* require new technology.
COVID19 arrived just as Moderna and BioNTech were in the midst of researching the utility of their mRNA platform. Funders and researchers had spent been working on mRNA vaccines since 2005, but none progressed to late-phase trials, as covered below
statnews.com/2020/11/10/the…
statnews.com/2020/11/10/the…
Thus companies were highly motivated to show their new (and proprietary) mRNA vaccines could work. This is not a bad thing; indeed patents exist to encourage investment and technology development. Pfizer BioNTech didn't even need government money to fund their vaccine trials.
This also explains why the West didn't produce WKV vaccines *in addition to* mRNA vaccines. That is, no Western company stood to profit from WKV. Fortunately Sinovac and Sinopharm did have motivation to pursue WKV vaccines. They provided a backup in case newer tech didn't work.
But it's concerning how narrowly we dodged the bullet here. If the mRNA vaccines had failed, the West would have to go begging to Sinovac and Sinopharm for doses, or wait for clearer and better results from the adenovirus vaccines (AstraZeneca, Sputnik).
Or given the limiting amount of mRNA vaccine doses, we can say we actually didn't dodge the bullet after all. If we had done both WKV and mRNA, we might be in a better situation now. Minus corporate motivation, WKV would have required government participation.
In fact, in March I wrote to a colleague who was corresponding with NIH and FDA on vaccine approaches, "Would Salk-style inactivated or whole killed virus vaccine (WKV) be worth considering as well? Its distribution and storage should be easier than a mRNA vaccine..."
"...and there is already evidence from SARS WKV that it induces neutralizing antibodies, can reduce viral titers in animal models, and is safe in humans." But as it became apparent CDC couldn't tie its own shoes and wasn't given any authority to do anything anyway, I dropped it.
The results now being announced for Sinovac and Sinopharm vaccines indeed suggests that what worked for SARS1 will work for SARS2 (COVID19) as well.
Thus these different approaches show, interestingly, how corporate motives now influence how we pursue public health in the West.
Thus these different approaches show, interestingly, how corporate motives now influence how we pursue public health in the West.
Happy to learn from @ProfTomEllis that there is one Western company testing a WKV vaccine, Valneva. They just started Phase 1/2 last week, which explains why I hadn't heard about them before. valneva.com/press-release/…
Sorry wrong reference; this is the correct one for Graham/McLellan/Moderna's MERS work (and it was in rabbit not mouse)
cen.acs.org/pharmaceutical…
cen.acs.org/pharmaceutical…
• • •
Missing some Tweet in this thread? You can try to
force a refresh