
1. Our COVID-19 paper on Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection is out today in @ScienceMagazine !
science.sciencemag.org/content/early/…
@ljiresearch @SetteLab @Dani6020
science.sciencemag.org/content/early/…
@ljiresearch @SetteLab @Dani6020
2. The immune system can remember viruses. And there are multiple different parts of the immune system that can remember a virus in different ways, so the immune system can fight the virus in multiple ways. 

3. Our data shows that the body’s immune system remembers novel coronavirus for at least 8 months after COVID-19, and multiple different branches of the immune system remember the virus. 

4. This suggests that the immune system can remember the SARS-CoV-2 virus for years, and most people may be protected from severe COVID-19 for substantial time. Which is certainly good news! 

5. For the aficionados, we found complex relationships between the different components of immune memory. 
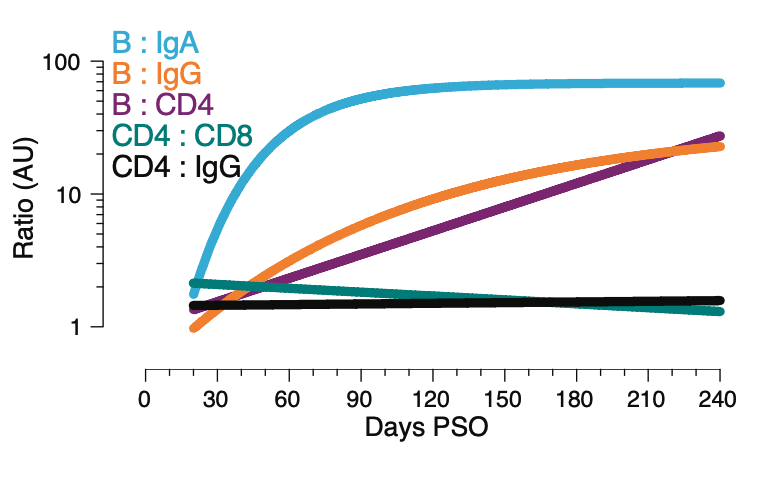
6. Which included a lack of predictive power of serum antibody titers for predicting the magnitude of the T cells. 

7. These overall COVID-19 immune memory findings are consistent with multiple other labs, including @PepperMarion, Nussenzweig lab, and van Zelm lab, and the healthcare workers study finding no symptomatic re-infections over 6 months. medrxiv.org/content/10.110…
8. We first reported this work as a pre-print in November, as a 6 month study, which has been expanded to 8 months, and additional data.
https://twitter.com/profshanecrotty/status/1328760517184212993?s=20
• • •
Missing some Tweet in this thread? You can try to
force a refresh






