
Press conference on results of Brazilian trial of CoronaVac, Sinovac's inactivated vaccine, cleared up the efficacy data & made the complexity of comparing a trial in healthcare professionals with others *really* clear: are they better at self-diagnosing Covid-19 symptoms?...1/n
...That's a central question now: does this trial capture more much "milder" infections than the others we've seen? They're suggesting it does & that drives down the apparent efficacy rate. Here's how the data plays out...2/n
...The trial's definition of symptomatic Covid-19: the first is a slide from the press conference (Portuguese), the second is from the English version of the trial protocol. The primary efficacy endpoint is regardless of whether the person had previous SARS-Cov-2 infection... 3/n 



...First the data for the results they reported at the previous press conference. The data are for 9,242 participants. For people needing hospitalization or having severe Covid-19, there were 0 cases with CoronaVac, ...4/n 
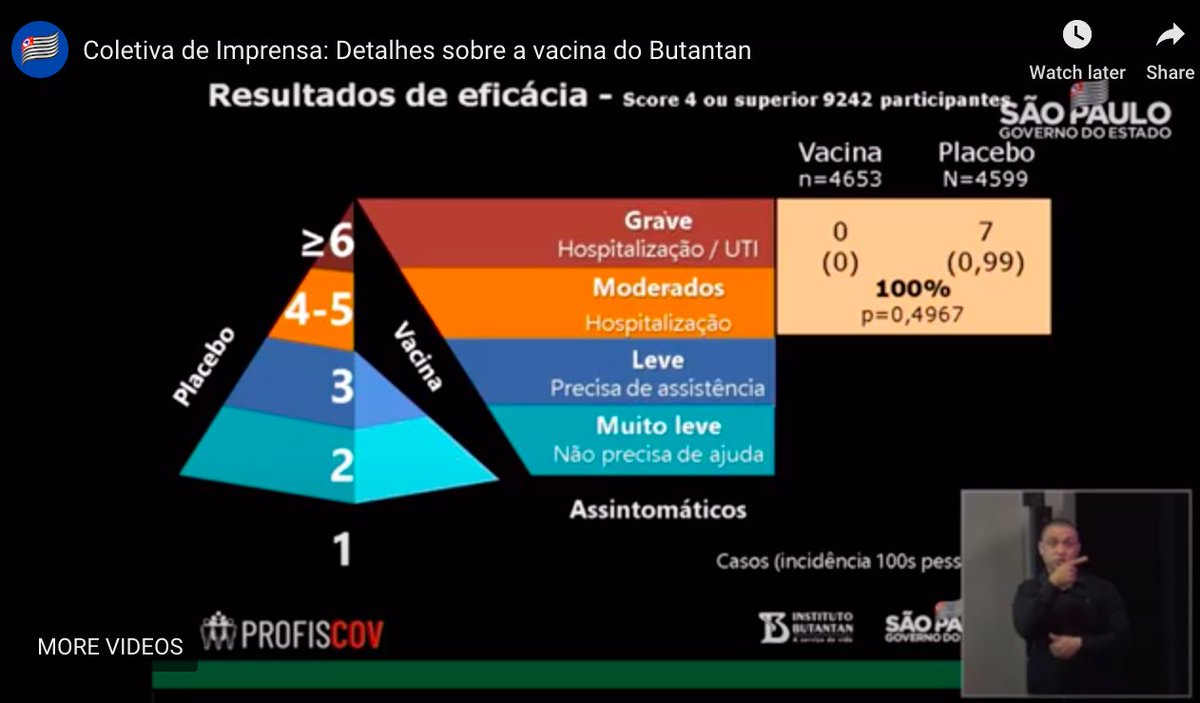
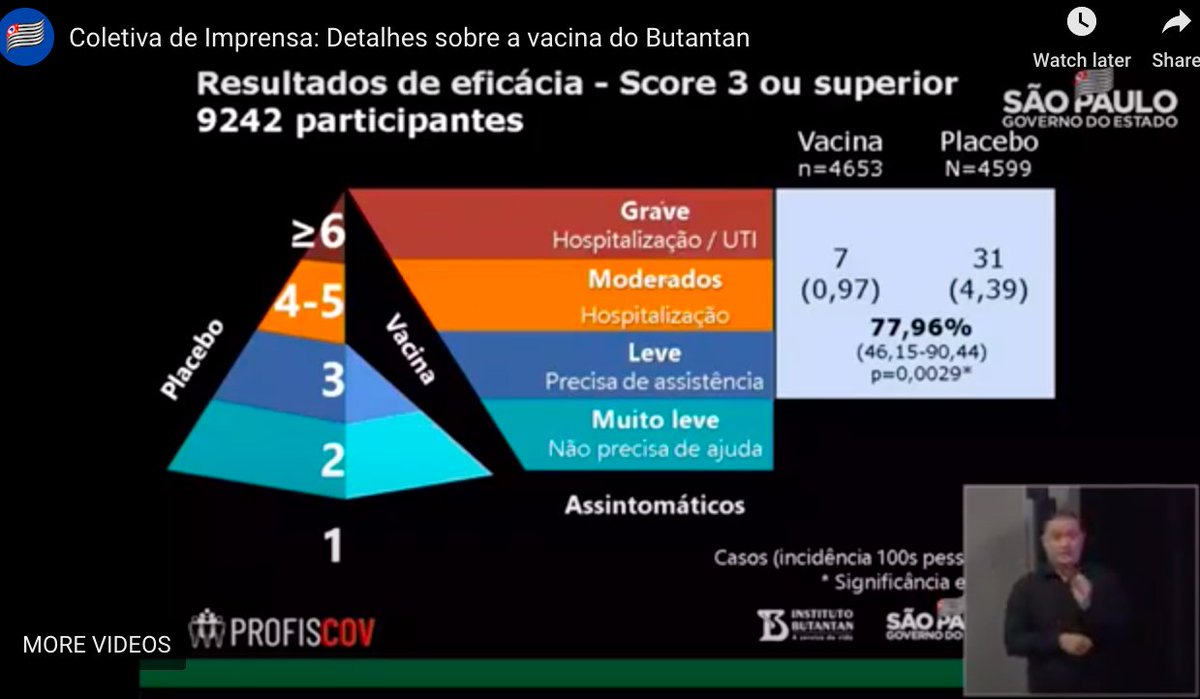
...Next level is the one they reported last time, the 78% efficacy. It wasn't the primary efficacy outcome. It was for people who needed medical assistance as well as the people hospitalized or severely ill: with a confidence interval from 46% to 90% - a lot of uncertainty...5/n 
...This is new: the overall efficacy. They believe while they don't have asymptomatic infections, they have tested for & captured more symptomatic infections than others could. That's a vaccine efficacy rate of 50.38%, CI of 35-62%. So just scraping over the WHO requirement...6/n 
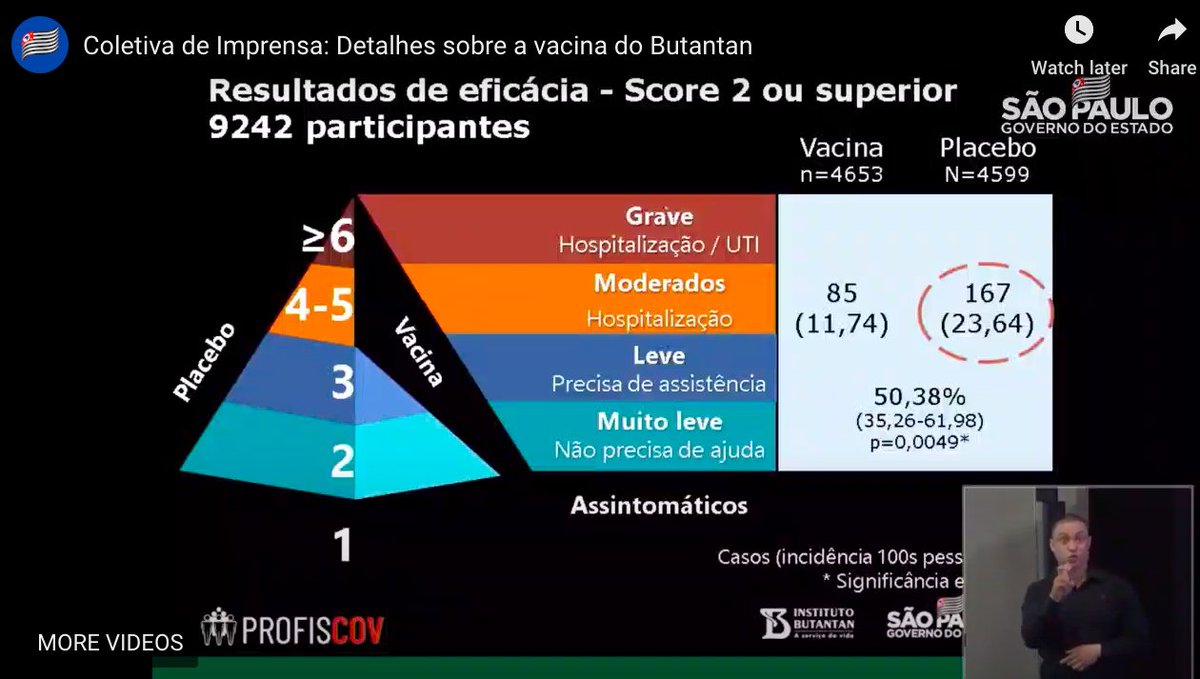
...How does this compare? I don't think the press conference revealed age split, but someone who speaks Portuguese might be able to say. Protocol set an overall target of about 10% of people over 60. Oxford/AstraZeneca came in at ~60% efficacy for up to age 55. Which means...7/n
...Brazilian trial, roughly same size & similar-ish confidence intervals, has more people at risk of (severe) Covid-19 & less likely to have strong immune response. Hard to know if it's similar, lower or higher than Oxford. We don't know the efficacy over time for CoronaVac...8/n
...Where does this leave us? I think they made an important point about self-diagnosis of symptoms in a healthcare worker (HCW) trial. The Turkish trial is also for HCWs, similar size, but we don't have the protocol for it, so don't know how otherwise similar...9/n
...Explanation for a previous tweet: when I said "similar CIs", I meant the range was as wide, not the same actual results.
Sources for thread: screenshots of slides from the press conference gauchazh.clicrbs.com.br/saude/noticia/… Protocol for the Brazilian trial: trialsjournal.biomedcentral.com/articles/10.11… 10/10
Sources for thread: screenshots of slides from the press conference gauchazh.clicrbs.com.br/saude/noticia/… Protocol for the Brazilian trial: trialsjournal.biomedcentral.com/articles/10.11… 10/10
...PS (always at least 1!!!): This time it's about the numbers. The number for the 2 groups in the slides they gave don't add up to the 9,242 in the slides: they add up to 9,252. I think this means we have to wait for a proper report to be *really* sure about the numbers.
..And here's a good English report of the press conference: bloomberg.com/news/articles/…
...About the Brazilian trial for CoronaVac (Sinovac vaccine) for those asking. If participants reported symptoms of Covid-19, they were tested: but if they didn't report symptoms, they weren't.
• • •
Missing some Tweet in this thread? You can try to
force a refresh








