
COVAXIN Efficacy: A thread...
n0
n0
Q1: Is there a method to hypothesise the efficacy of COVAXIN when no data has been released?
Q2: How is this efficacy enhanced & measured?
Q3: Why can’t COVAXIN be compared with other mRNA vaccines like COVISHIELD (AstraZeneca), ModeRNA and Pfizer?
n1
Q2: How is this efficacy enhanced & measured?
Q3: Why can’t COVAXIN be compared with other mRNA vaccines like COVISHIELD (AstraZeneca), ModeRNA and Pfizer?
n1
We know that COVAXIN, also known as BBV152, is based on a tested technology which inactivates or weakens the whole virus, in this case, SARS-CoV-2. NIV isolated the virus strain (NIV-2020-770) of the coronavirus from a COVID-19 patient and sequenced its genome.
n2
n2

The genome sequencing revealed a shift mutation Asp614Gly of aspartic acid to glycine at the amino acid position 614 of the spike protein (SP). This SP receptor binding domain structure determines the attachment of the virus on the surface of the human cells via ACE2 receptor
n3
n3
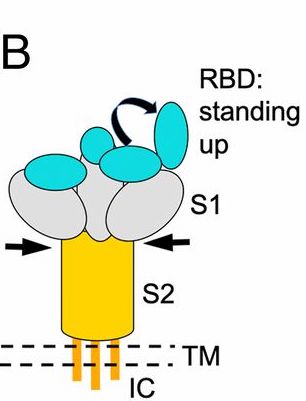
An explanation of the mechanics of the receptor binding structure of spike protein affects viral entry by attachment is below (See image description)
n4
n4
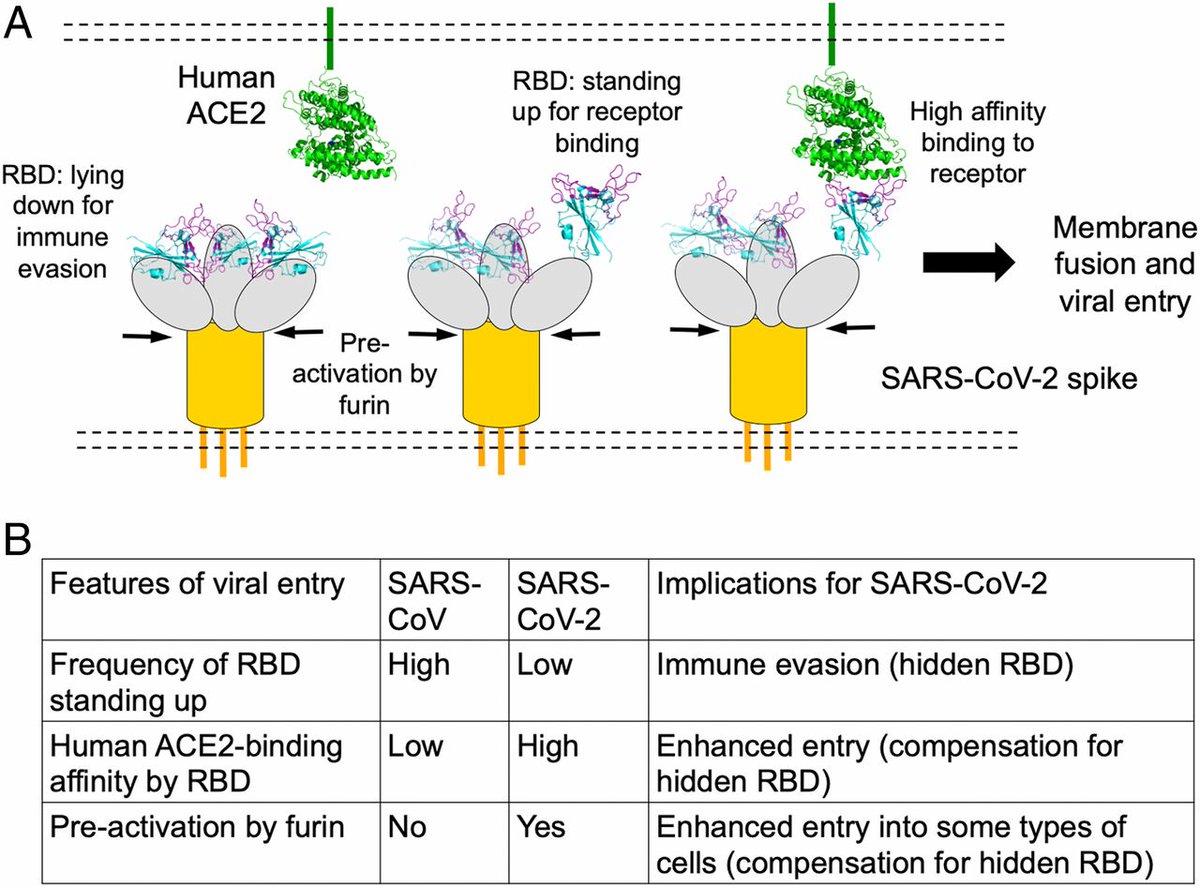
.@BharatBiotech acquired this strain & prepared a whole-virion SARS-CoV-2 vaccine by inactivating it with β-propiolactone - a reagent used to inactivate virus without degrading its proteins.
In Sept 2020, Virovax licenced its adjuvant Alhydroxiquim-II
n5
thelancet.com/journals/lanin…
In Sept 2020, Virovax licenced its adjuvant Alhydroxiquim-II
n5
thelancet.com/journals/lanin…
This Virovax adjuvant is historically known to enhance antibody production. Thus, COVAXIN, a whole virion with its intact spike protein, receptor-binding domain, and the nucleocapsid protein of SARS-CoV-2 has the ability to potentially produce stronger IgG responses. Great...
n6
n6
Thus, other vaccines are different from this whole virion inactivated adjuvant tech
1. mRNA (ModeRNA & Pfizer/BioNTech)
2. Recombinant protein (Novavax)
3. Replication Deficient Vector (CanSino Ad5 nCoV, Sputnik V or Gam-COVID-Vac, AstraZeneca ChAdOx1 nCoV-19, J&J / Janssen)
n7
1. mRNA (ModeRNA & Pfizer/BioNTech)
2. Recombinant protein (Novavax)
3. Replication Deficient Vector (CanSino Ad5 nCoV, Sputnik V or Gam-COVID-Vac, AstraZeneca ChAdOx1 nCoV-19, J&J / Janssen)
n7
Due to such varied tech, the only vaccines worth comparing with COVAXIN are
1. Sinovac' s CoronaVac
2. Sinopharm with WIV04 strain in AL adjuvant
3. Sinopharm's with HB02 strain in AL adjuvant or BBIBP-CorV
n8
1. Sinovac' s CoronaVac
2. Sinopharm with WIV04 strain in AL adjuvant
3. Sinopharm's with HB02 strain in AL adjuvant or BBIBP-CorV
n8
In vaccine efficacy, what is quantified?
Virus neutralizing GMTs OR
Geometric mean titre
The average antibody titre for a group of subjects calculated by multiplying all values and taking
the nth root of this number, where n is the number of subjects with available data.
n9
Virus neutralizing GMTs OR
Geometric mean titre
The average antibody titre for a group of subjects calculated by multiplying all values and taking
the nth root of this number, where n is the number of subjects with available data.
n9
Some other terms from the WHO guidelines
Immunogenicity
The capacity to elicit a measurable immune response.
Seroconversion
A predefined increase in serum antibody concentration or titre. (Let's say a certain value of GMT above the baseline titre)
n10
who.int/biologicals/ex…
Immunogenicity
The capacity to elicit a measurable immune response.
Seroconversion
A predefined increase in serum antibody concentration or titre. (Let's say a certain value of GMT above the baseline titre)
n10
who.int/biologicals/ex…
Let's see the data for each of the three vaccines to be compared
1. Sinovac conducted its trials in multiple countries and various numbers emerged questioning Coronavac's efficacy.
The latest report (20Jan 21) from Chile said 78% after 2doses (Agence France Presse)
n11
1. Sinovac conducted its trials in multiple countries and various numbers emerged questioning Coronavac's efficacy.
The latest report (20Jan 21) from Chile said 78% after 2doses (Agence France Presse)
n11
1. Sinovac's Coronavac contd...
However, other countries concluded with different results at various stages of testing.
-Brazil 50.4% on 13Jan 21
-Indonesia 65.3% on 10Jan 21
-Brazil 50-90% on 25 Dec 20
-Turkey 91.25% on 25 Dec 20
n12
However, other countries concluded with different results at various stages of testing.
-Brazil 50.4% on 13Jan 21
-Indonesia 65.3% on 10Jan 21
-Brazil 50-90% on 25 Dec 20
-Turkey 91.25% on 25 Dec 20
n12
The latest phase III clinical trials of Coronavac is below clinicaltrials.gov/ct2/show/NCT04…
clinicaltrials.gov/ct2/show/NCT04…
clinicaltrials.gov/ct2/show/NCT04…
and is expected to finish in May, April and Sept 2021 respectively
n13
clinicaltrials.gov/ct2/show/NCT04…
clinicaltrials.gov/ct2/show/NCT04…
and is expected to finish in May, April and Sept 2021 respectively
n13
2. Sinopharm's strain WIV04 & HB02 (BBIBP-CorV).
The manufacturing process of BBIBP-CorV is very similar to the other vaccine Sinopharm produced (WIV04), except that BBIBP-CorV used a different HB02 strain rather than WIV04 strain.
pubmed.ncbi.nlm.nih.gov/32778225/
n14
The manufacturing process of BBIBP-CorV is very similar to the other vaccine Sinopharm produced (WIV04), except that BBIBP-CorV used a different HB02 strain rather than WIV04 strain.
pubmed.ncbi.nlm.nih.gov/32778225/
n14
2. Sinopharm contd...
WIV04- grown in culture & isolated from a patient in Jinyintan Hospital, Wuhan, China.
Also used β-propiolactone inactivation and alum adjuvant enhancement like COVAXIN
n15
WIV04- grown in culture & isolated from a patient in Jinyintan Hospital, Wuhan, China.
Also used β-propiolactone inactivation and alum adjuvant enhancement like COVAXIN
n15
2. Sinopharm contd..
Also conducting phase 3 clinical trials in various countries,
clinicaltrials.gov/ct2/show/NCT04…
chictr.org.cn/showprojen.asp…
clinicaltrials.gov/ct2/show/NCT04…
clinicaltrials.gov/ct2/show/NCT04…
Expected to finish phase 3 by Sep-Dec 2021
n16
Also conducting phase 3 clinical trials in various countries,
clinicaltrials.gov/ct2/show/NCT04…
chictr.org.cn/showprojen.asp…
clinicaltrials.gov/ct2/show/NCT04…
clinicaltrials.gov/ct2/show/NCT04…
Expected to finish phase 3 by Sep-Dec 2021
n16
2. Sinopharm contd
Efficacy:
On 29 Dec 2020, Sinopharm announced 79.34% efficacy.
However, UAE media reports have stated 99% seroconversion rate of neutralizing antibody and 100% effectiveness in preventing moderate and severe cases of the disease.
n17
reuters.com/article/health…
Efficacy:
On 29 Dec 2020, Sinopharm announced 79.34% efficacy.
However, UAE media reports have stated 99% seroconversion rate of neutralizing antibody and 100% effectiveness in preventing moderate and severe cases of the disease.
n17
reuters.com/article/health…
It looks like the efficacy of the same vaccine can differ.
Other than the GMTs, What are some of the factors that define efficacy?
1. The pathogen,
2. Consequences of infection, and
3. Transmission dynamics
n18
thelancet.com/journals/lanin…
Other than the GMTs, What are some of the factors that define efficacy?
1. The pathogen,
2. Consequences of infection, and
3. Transmission dynamics
n18
thelancet.com/journals/lanin…
If the factors like the virus and its mutations, infection consequences and rate of transmission are extremely dynamic.
To hypothesize vaccine outcomes by looking at other similar vaccines, then the comparison of evoked neutralising antibody titres (GMT) makes sense?
n19
To hypothesize vaccine outcomes by looking at other similar vaccines, then the comparison of evoked neutralising antibody titres (GMT) makes sense?
n19
See the figure below,
GMTs of the two vaccines discussed above in phase III trials of various doses.
Clearly, the 3 dose Sinopharm has the highest (close to 300 GMT) with its triple dose 2.5 and 10 microgm
GMTs of the two vaccines discussed above in phase III trials of various doses.
Clearly, the 3 dose Sinopharm has the highest (close to 300 GMT) with its triple dose 2.5 and 10 microgm
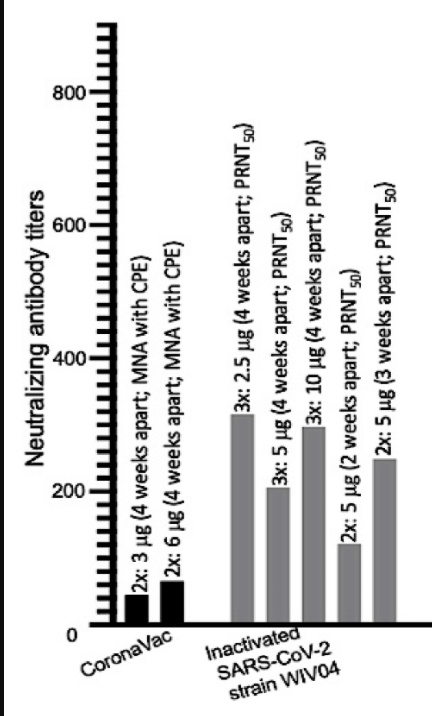
Will these titres would last long enough (in excess of 6-12 months)?
We need to wait and see more results before making definite conclusions about efficacy. However, in phase 3, waiting 30-45 days after the last dose should be good enough, especially for adverse events.
n21
We need to wait and see more results before making definite conclusions about efficacy. However, in phase 3, waiting 30-45 days after the last dose should be good enough, especially for adverse events.
n21
But despite the huge variation, there could be a decent amount of GMT evoked with COVAXIN as it is very close to the other inactivated vaccine in preparation except some minor SP mutation.
But is that good enough? Yes
Are there other vaccines that might be better? Yes
n22
But is that good enough? Yes
Are there other vaccines that might be better? Yes
n22
Will the other vaccines of different technologies provide the same protection against new mutations?
The whole inactivated virion like COVAXIN might provide better protection but there is no data.
Should you take the vaccine? yes, but cautiously.
No, for severe allergies
n23
The whole inactivated virion like COVAXIN might provide better protection but there is no data.
Should you take the vaccine? yes, but cautiously.
No, for severe allergies
n23
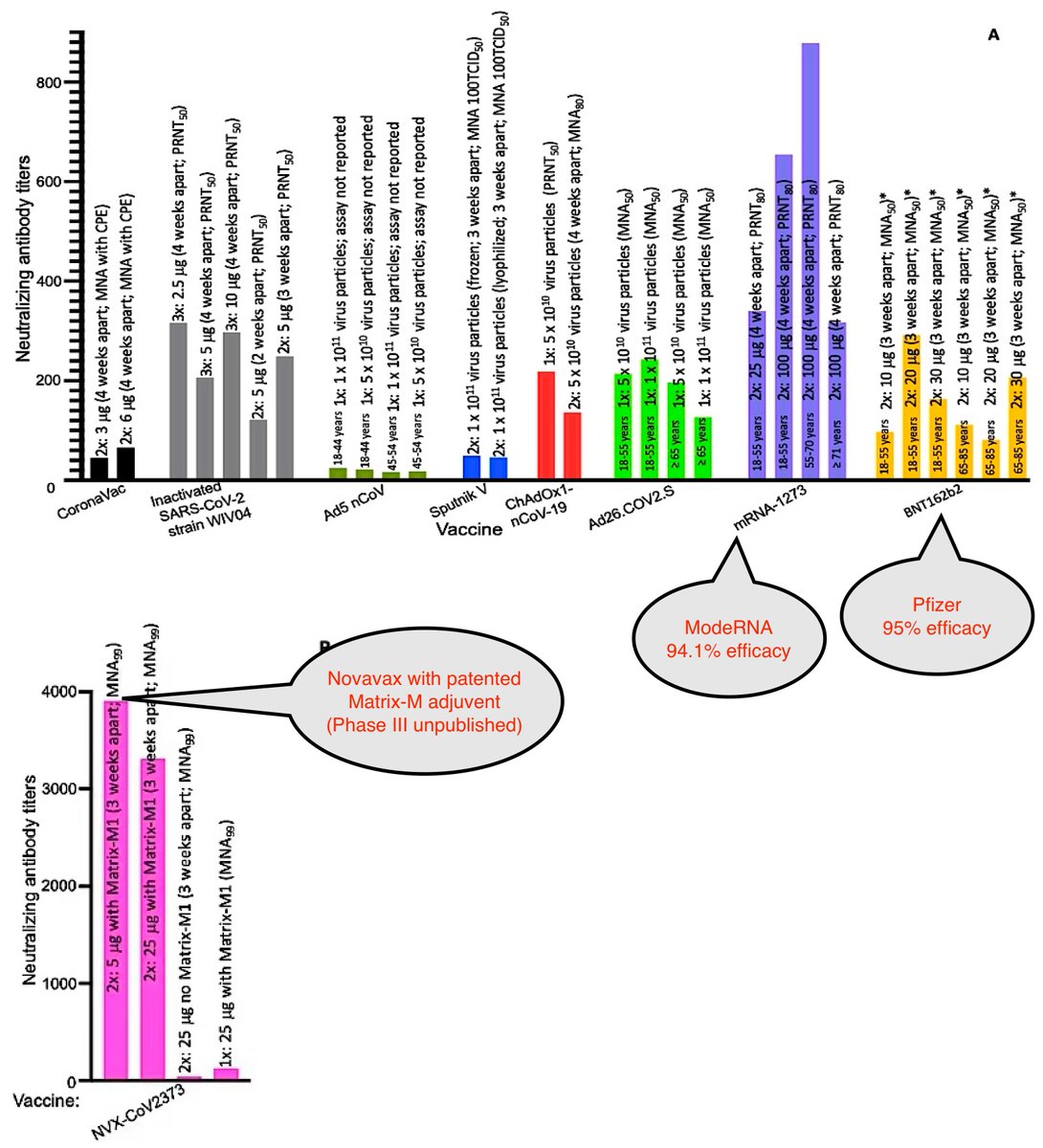
Let's compare the GMTs of other vaccines with Sinopharm's CoronVac & Sinopharm's WIV04. (See labels below)
Phase III data:
Highest GMTs for published data ModeRNA & Pfizer
Highest overall Novavax
mdpi.com/1999-4915/13/1…
n24//
Phase III data:
Highest GMTs for published data ModeRNA & Pfizer
Highest overall Novavax
mdpi.com/1999-4915/13/1…
n24//
Correction:
Q3: Why can’t COVAXIN be compared with COVISHIELD (AstraZeneca) and other mRNA vaccines like Gennova, ModeRNA and Pfizer?
Q3: Why can’t COVAXIN be compared with COVISHIELD (AstraZeneca) and other mRNA vaccines like Gennova, ModeRNA and Pfizer?
• • •
Missing some Tweet in this thread? You can try to
force a refresh



