
It's on the agenda this week: possibly a decision on the Oxford/AstraZeneca vaccine from EMA's (Euro drug regulator) Human Medicine Committee? Applicants will be questioned at the meeting.
(EMA process steps explained here: ema.europa.eu/en/human-regul…)
(EMA process steps explained here: ema.europa.eu/en/human-regul…)
https://twitter.com/EMA_News/status/1353643777366216704
Last night, I tweeted ⬆️ that the EMA (Euro drug regulator) meeting was considering the Oxford/AstraZeneca vaccine. I included a link to EMA processes, because the language on the agenda suggested there could be a problem: this is the explanation I mean in that link ....1/n 

...German newspapers now reporting a claim the efficacy rate for people over 65 is only 8% & approval is indeed in question for that age group, at least (Paywalls: I can't read in full.) Rumor at this point, but a limit ...2/n bild.de/bild-plus/poli…, handelsblatt.com/politik/deutsc…
...on people >65, if that comes, would not surprise me. Here's an excerpt from my December vaccine roundup: absolutelymaybe.plos.org/2020/12/20/why… What do we know about both these issues, efficacy & safety in over 65s? ....3/n 

...Here's age breakdown in Oxford paper on vaccine efficacy: <500 people aged 70+, & just over 1,400 over the age of 55. Efficacy was only analyzed for up to the age of 55: there just aren't enough people to know how this works in people over 55 ...4/n thelancet.com/journals/lance… 
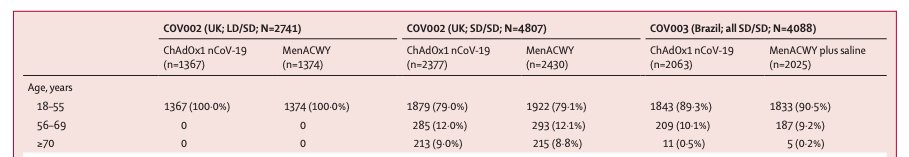
...Unlike some other vaccines, early phase research for this vaccine didn't include older people either. Which means there's no safety data to add, either. Because the numbers are so small in people 65+, an efficacy calculation wouldn't mean much...5/n
...Uncertainty around an estimate of vaccine efficacy in a group that could be quite a bit under 1,000 people would be huge: could fluctuate dramatically over time. For comparison, here's what I said in that post about the 2 mRNA vaccines (with thousands of older people)...6/n 
...Another example: Sputnik V. Anmat, Argentina's drug regulator, originally didn't approve it for people over 60 because of lack of data. Changed recently when Sputnik V provided antibody data plus an efficacy estimate of 92% (67-98%) in 2,144 people..7/n
https://twitter.com/hildabast/status/1352027992436793345
...One thing has changed since the report of the efficacy data though: the UK has been vaccinating a lot of elderly people using this vaccine. So although it's very short-term & mostly a single shot so far, that's considerable extra safety experience now... 8/9
...My opinion? Would I be surprised if EMA didn't approve it for people >65? No. Until US trial reports, we don't know the impact on older people. Do I believe the efficacy rate could be only 8%? Risk of an efficacy calculation in such a small group being a fluke is too high. 9/9
...PS: Dug into the MHRA report on Oxford AstraZeneca vax. Reported 1,169 people aged 65+ (don't know how many of those exactly 65) got the vax, counting all dosing regimens & studies (not just the efficacy subgroup). EMA reportedly has more recent data. assets.publishing.service.gov.uk/government/upl…
...Reuters reporting that the German Ministry of Health is implying that someone basically misunderstood something: data don't show an 8% efficacy rate.
https://twitter.com/AndyMacaskill/status/1354002361543495681EMA committee due to make a decision on Friday.
...Handelsblatt rebutted German Ministry of Health's rebuttal of their report about efficacy of the Oxford/AstraZeneca vaccine in older people. Their sources insist no one messed up numbers. This time, not behind a paywall. handelsblatt.com/politik/deutsc… Hope EMA is clear on this & soon
...Another instalment in Oxford/AstraZeneca "he said, she said" saga. AstraZeneca chief says efficacy similar for younger/older, wide confidence intervals. Says he doesn't know the efficacy rate, but there are now 200 events (that's double the original)... repubblica.it/cronaca/2021/0…
...Also says supply problems are because 1 large Euro facility having difficulty making enough of the vaccine (Australia's CSL is too). UK facility did too early on, but that's resolved. They modified the formula (?): watch for this issue in EMA report too. (HT: @MaffewEC) 

• • •
Missing some Tweet in this thread? You can try to
force a refresh







