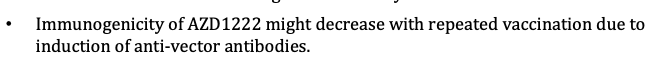This Oxford-AstraZeneca vaccine study from a preprint a couple of weeks ago is now published in the Lancet. At first look, there are some additions... 1/n thelancet.com/journals/lance…
https://twitter.com/hildabast/status/1356707913310474241
...In the original thread, I'd had a question about hospital admissions, but ignore that if you re-read it: the wording is expanded a little in the paper, & it clears it up... 2/n
...The supplementary info includes listing of serious adverse events & unsolicited adverse events, but no data on solicited adverse events - all of that is new...3/n thelancet.com/cms/10.1016/S0…
...The limitations section now includes relevant differences in the group getting only 1 dose (the data were included in the preprint, but it wasn't highlighted in this way as the important limitation that it is)...4/5 

...The data appear to be the same, but I didn't compare carefully. (There are presentation differences eg vaccine efficacy data now goes to a decimal point.)...5/5
• • •
Missing some Tweet in this thread? You can try to
force a refresh