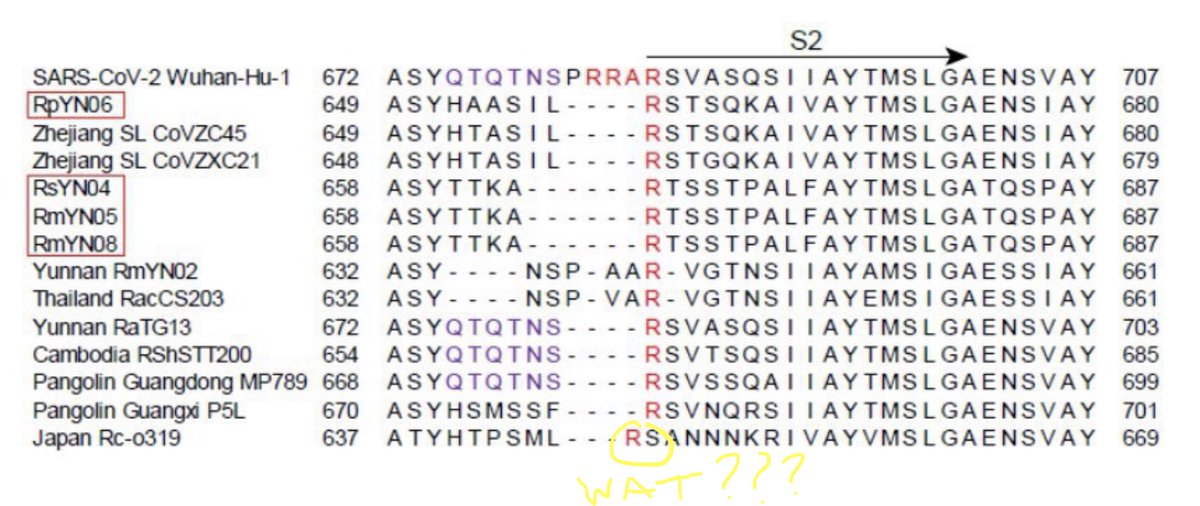
1/n
Ok, let me try and harp on my evolutionary, well, harp 😂 And maybe my favorite EvoBio couple, @HeatherEHeying and @BretWeinstein can smack me on the head if say something dumb.
So basically infectivity and virulence/pathogenicity are two different properties of a virus,
Ok, let me try and harp on my evolutionary, well, harp 😂 And maybe my favorite EvoBio couple, @HeatherEHeying and @BretWeinstein can smack me on the head if say something dumb.
So basically infectivity and virulence/pathogenicity are two different properties of a virus,
https://twitter.com/Blitzer55/status/1380844072370339845
2/n
which *might* be correlated but do not have to be. First of all, we must understand: these little viruses are not out to kill us. All they “want” is to replicate. And want is in quotes because of course they don’t have any agency:
which *might* be correlated but do not have to be. First of all, we must understand: these little viruses are not out to kill us. All they “want” is to replicate. And want is in quotes because of course they don’t have any agency:
3/n
they are just a self-replicating collection of molecules, set in motion by some random LUCA prime mover billions of years ago. So the pathogenicity/virulence of a virus is really an unwanted side-effect for both parties:
they are just a self-replicating collection of molecules, set in motion by some random LUCA prime mover billions of years ago. So the pathogenicity/virulence of a virus is really an unwanted side-effect for both parties:
4/n
neither we nor the virus want us to die and stop propagating it. But sometimes the virus is too effective at hijacking our cells that it messes up our ability to do something critical to our survival, like breathing.
neither we nor the virus want us to die and stop propagating it. But sometimes the virus is too effective at hijacking our cells that it messes up our ability to do something critical to our survival, like breathing.
5/n
And at other times it is actually our immune system that is so effective at killing the virus that it kills us in that good fight as well 🥲
And at other times it is actually our immune system that is so effective at killing the virus that it kills us in that good fight as well 🥲
6/n
But back to the evolutionary aspects of a new viral strain. I think it is pretty self-evident that with time a novel virus strain would “benefit” from decreasing in pathogenicity. Orthogonally, it *should* benefit from increasing in transmissibility (infectiveness).
But back to the evolutionary aspects of a new viral strain. I think it is pretty self-evident that with time a novel virus strain would “benefit” from decreasing in pathogenicity. Orthogonally, it *should* benefit from increasing in transmissibility (infectiveness).
7/n
However, there is a complex dynamic at play here: if the virus is actually gaining in pathogenicity, a gain in transmissibility could be bad for its long-term survival, because it would quickly run out of hosts.
However, there is a complex dynamic at play here: if the virus is actually gaining in pathogenicity, a gain in transmissibility could be bad for its long-term survival, because it would quickly run out of hosts.
8/n
So the best long-term survival adaptation strategy for a novel strain is to gain in transmissibility while decreasing in pathogenicity. That said, there are also no guarantees that (a) this is possible and (b) this strategy would be found.
So the best long-term survival adaptation strategy for a novel strain is to gain in transmissibility while decreasing in pathogenicity. That said, there are also no guarantees that (a) this is possible and (b) this strategy would be found.
9/n
Viruses try to “find” these strategies blindly (recall the Blind Watchmaker) — they just incur novel mutations and the ones that provide some sort of an advantage tend to stick around.
Viruses try to “find” these strategies blindly (recall the Blind Watchmaker) — they just incur novel mutations and the ones that provide some sort of an advantage tend to stick around.
10/n
And “advantages” could change in the course of the pandemic. A spike mutation that would not have provided any advantage early on, could suddenly make a virus less sticky to antibodies that were developed to the previous strain, thereby paving the way to reinfection ability
And “advantages” could change in the course of the pandemic. A spike mutation that would not have provided any advantage early on, could suddenly make a virus less sticky to antibodies that were developed to the previous strain, thereby paving the way to reinfection ability
11/n
In any case, let’s just hope we can stamp out SARS2 quickly before it has enough time to find one of many possible novel ways of escaping our immune memory from vaccines or previous infections.
In any case, let’s just hope we can stamp out SARS2 quickly before it has enough time to find one of many possible novel ways of escaping our immune memory from vaccines or previous infections.
• • •
Missing some Tweet in this thread? You can try to
force a refresh