
The evidence assessments - but not the WHO decisions - for Sinopharm's Beijing vax & Sinovac's CoronaVac are going up on the WHO website who.int/news-room/even… HT @lutl88 @hvasquezhg ...1/n
...Sinopharm Beijing: WHO assessment. 45,000-participant trial in UAE/Bahrain/Egypt/Jordan, doses 21 days apart, efficacy:
- Overall: 78.1% (CI 65-86)
- For people with no previous infection: 80.8% (67-89) (the usual efficacy rate for others)
cdn.who.int/media/docs/def… ...2/n
- Overall: 78.1% (CI 65-86)
- For people with no previous infection: 80.8% (67-89) (the usual efficacy rate for others)
cdn.who.int/media/docs/def… ...2/n
...- Efficacy against hospitalization: 78.7% (CI 26-94)
- 1 death only, in the placebo group
But this is only up to age 60: negligible people over 60, & none had Covid-19 (in vax or placebo group)
...3/n
- 1 death only, in the placebo group
But this is only up to age 60: negligible people over 60, & none had Covid-19 (in vax or placebo group)
...3/n
... Safety data for 16,671 people who got Beijing vax (45,000 includes an arm for Wuhan vax, not reported here). The rate of severe adverse events (grade 3) wasn't higher in the vax group - nor was the rate of serious adverse events (SAEs). Only 2 SAEs possibly vax-related...4/n 

... Although can't assess vaccine efficacy for older people, they cite safety data from China in older people, where 1.1 million have been injected. (That doesn't really substitute for clinical trial data, though)...5/n
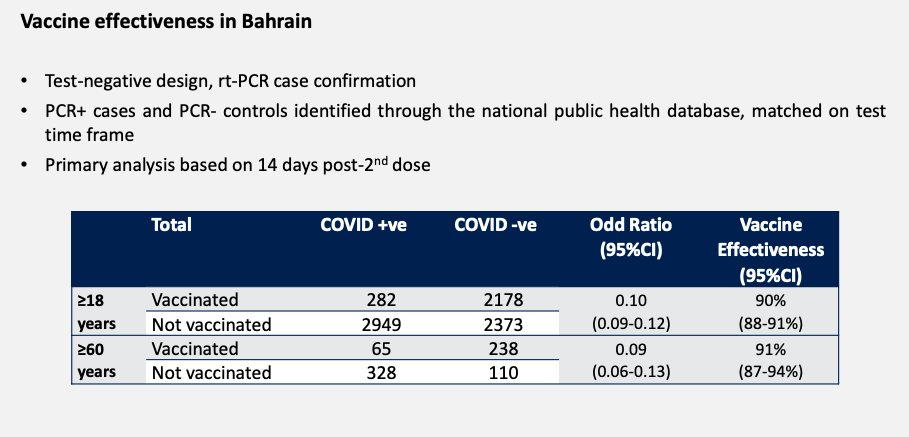
...Not much data here, but this is impressive. It's a test-negative control study from Bahrain. Vaccine efficacy 14 days after 2nd dose:
- All adults: 90% (88-91)
- 60+: 91% (87-94)
...6/n
- All adults: 90% (88-91)
- 60+: 91% (87-94)
...6/n
...Another thing to take into account for the phase 3 trial: lacked people with co-morbidities (except BMI over 30).
Conclusion on variants of concern: laboratory study showing protection but reduced signs of immunity for B.1.351 ("SA"), but no clinical data on variants ...7/n
Conclusion on variants of concern: laboratory study showing protection but reduced signs of immunity for B.1.351 ("SA"), but no clinical data on variants ...7/n
..Summary of what's missing from the evidence: hospitalization data, but not data on severe disease.
Conclusion: "High level of confidence" that 2 doses protect against Covid-19 in people aged 18 to 59, with "moderate level of confidence" that adverse events are low for them..8/n
Conclusion: "High level of confidence" that 2 doses protect against Covid-19 in people aged 18 to 59, with "moderate level of confidence" that adverse events are low for them..8/n

... Side note: Just in case anyone gets confused by this - they have the wrong description on a link that says it's for China National Biotech Group (Sinopharm subsidiary) - in fact, that leads to a Sinovac presentation...9/n
... So there's nothing more on the Sinopharm Beijing vax so far then. Bottom line? The trial plus the Bahrain case-control study add up to results that are actually better than we've been led to expect for this vaccine. & we sure could use *that* good news...10/n
...On to WHO evidence assessment for Sinovac's CoronaVac cdn.who.int/media/docs/def… (And also note, that incorrectly labeled file has "confidential" on it, so I for one have downloaded it! cdn.who.int/media/docs/def… ) ...11/n
...They say 260 million doses have been distributed.
Here's the efficacy data they're going on: most of this is already public. Again, negligible data for 60+ in the trials: Brazil, Indonesia, Turkey - with a 2-week dosing interval...12/n
Here's the efficacy data they're going on: most of this is already public. Again, negligible data for 60+ in the trials: Brazil, Indonesia, Turkey - with a 2-week dosing interval...12/n

... As well as the trials, they're taking 2 other studies into account. The Chile study (which I tweeted about here 
https://twitter.com/hildabast/status/1383183542495039490) & a test-negative case control study from Brazil, which is a first sighting...13/n

..Efficacy for symptomatic Covid-19 (previous table) is range we already know: 14 days between doses, ~50% in healthcare worker trial in Brazil & when P1 ("Brazil" variant of concern dominates, 84% in general population in Turkey. Closer to 70% for 28 day interval in Chile...14/n
...High rate of co-morbidity in the healthcare worker trial in Brazil: they put it at 56%
High rate of protection against hospitalization, but not enough data for certainty on how high that is
Definitions of symptomatic Covid-19 differed between the 3 trials unfortunately..15/n
High rate of protection against hospitalization, but not enough data for certainty on how high that is
Definitions of symptomatic Covid-19 differed between the 3 trials unfortunately..15/n
...Safety profile good again. Again, as you'd expect from an inactivated vaccine, low rate of adverse events: severe & serious adverse events weren't increased in the vax group in the trials. Again, though, usage in older people is outside the trials ...16/n 

...Results shown from several community studies in Chile & Brazil - none of these are new. Summary on variants of concern: for this vaccine, we've seen it up against P1 in those countries recently...17/n 

...They formulated the evidence gaps a little differently - but really the data presented here doesn't seem all that different to Sinopharm's Beijing vax
Same level of confidence on the evidence for people aged 18 to 59 as for Beijing vax ...18/n
Same level of confidence on the evidence for people aged 18 to 59 as for Beijing vax ...18/n

...On to the Sinovac presentation marked confidential. And that logo, hey? cdn.who.int/media/docs/def… ...19/n 

...There's brief summaries of conclusions of preclinical data on reproductive safety that I don't think has been published (but my memory could be failing me on this)...20/n
...This is definitely new: phase 1/2 trial data on neutralizing antibodies, showing the results for the 3-17 yr age group: up to now, we've just seen the conclusion that their immune response was higher...21/n 
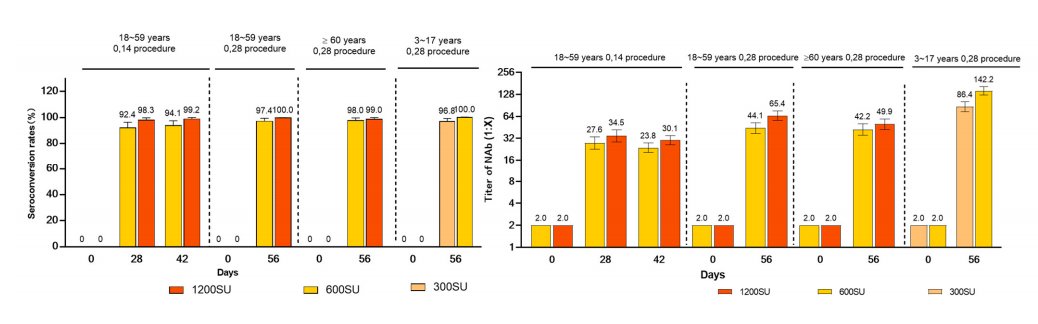
...This is also new: a bit more detail from the trial in Turkey (though it might all have been in the WHO's assessment). ...22/n 

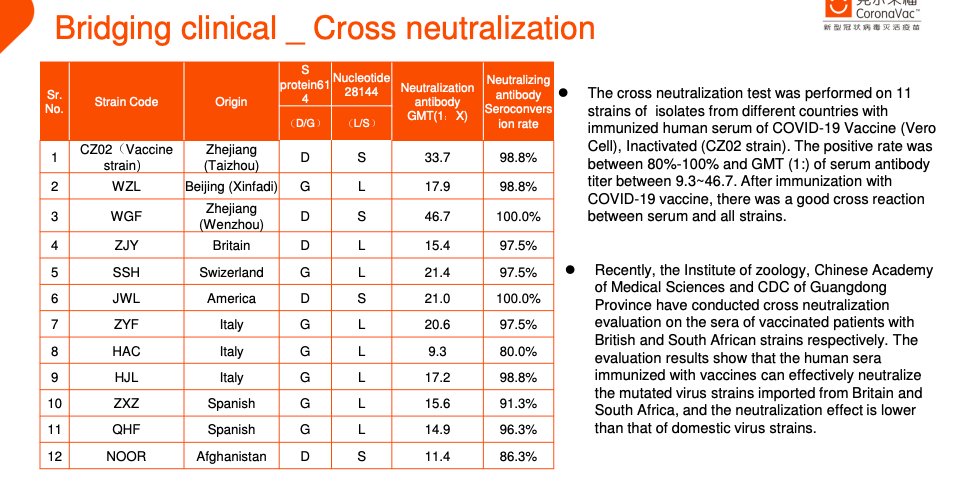
... I think this is new - there have been a couple of publications on variants of concern, but I don't think this is in there ...23/n 
...Ah, they say that of the 260m doses that have been shipped out, 160m have been administered.
...24/n
...24/n
...Very thin assessments from WHO, considering these vaxes haven't been through a major agency like EMA or FDA. But these vaccines work - & CoronaVac has been shown to still offer protection against P1 (another vax that maybe didn't bank on the right interval between doses) 25/25
• • •
Missing some Tweet in this thread? You can try to
force a refresh










