
#apaperaday Today’s pick is from @LiebertPub @OTSociety journal Nucleic Acid Therapeutics by Hammond et al from Matthew Wood’s group. It will be published in a special issue in NAT dedicated to “negative results”. Though of course with well done science any result moves science. 

The paper describes the use of alternatives for phosphorothioate (PS) as ASO backbones for splice switching ASOs. This backbone increases stability and cell uptake but leads to safety issues. Model ASO used here is nusinersen (spinraza, approved for spinal muscular atrophy).
As alternatives mesyl (methanesulfonyl phosphoroamidate) and busyl (L-butanesulfonyl phosphoroamidate) were evaluated. Hope is these modifications increase stability and reduce toxicity. For RNase H ASO these modifications showed increased potency in vitro. 
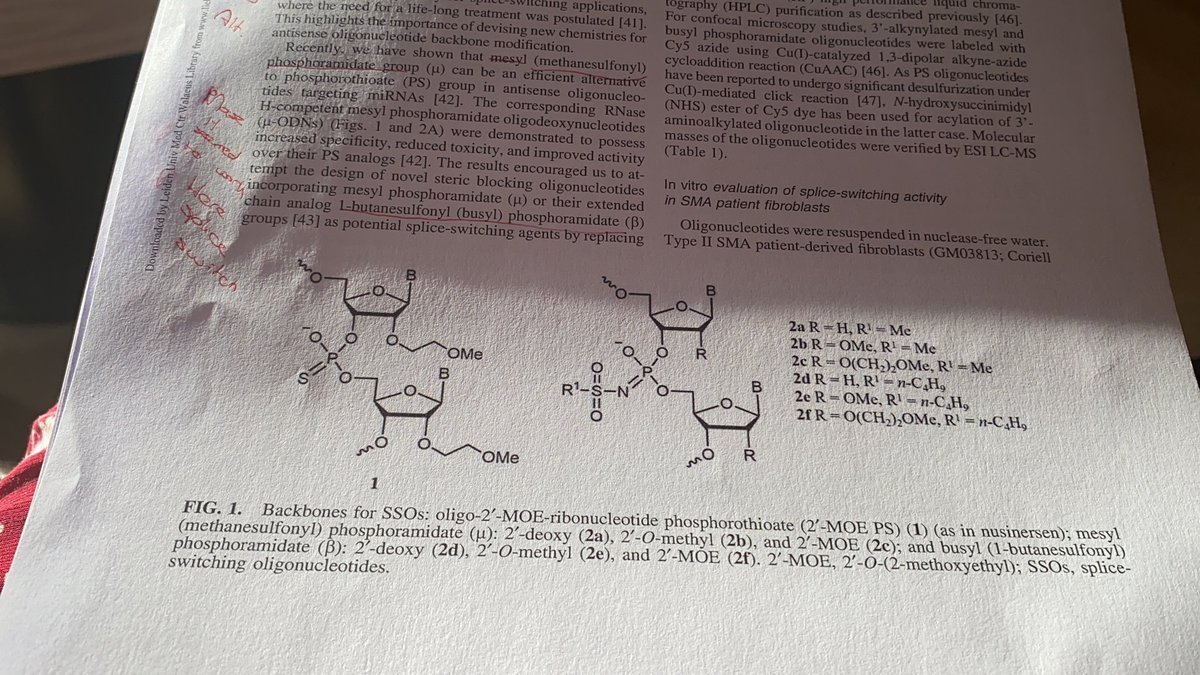
Authors compared mesyl busyl and PS backbones for DNA, OMe and MOE versions of nusinersen. DNA and scrambled ASOs were found not effective at increasing inclusion of SMN2 exon 7 in SMA patient fibroblasts. PS and mesyl were effective but besyl was not for MOE and OMe 

Authors studied intracellular distribution and endosomal escape in HEK293 cells using gymnosis (results previous tweet used lipofectamine). All modifications showed similar levels of escape. However busyl and mesyl were located mainly in cytosol. Splicing takes place in nucleus.
Finally authors tested in vivo potency of mesyl version vs nusinersen in SMA mouse model using SC injections of up to 40 mg/kg at day of birth. For nusinersen highest dose increased survival to median 222 days, mesyl to only 18 days (untreated 5-9 days).
Conclusion is that at least for nusinersen this modification is not an improvement in animal model. RNase H results were more promising but only in vitro so far. However there cytosol localization is less of a problem. Authors conclude more work is needed.
My comments: kudos for publishing something that does not show an improvement. This allows chemists to maybe build better alternatives and also prevents duplications of experiments because others thought to try these modifications.
Not sure why nusinersen was used as test compound. This one is delivered intrathecally and here the PS toxicity so far seems not to be an issue. However as a model system it works. Also not sure how well the HEK studies in vitro model what happens in vivo.
Finally a shout out to guest editors of this issue in NAT @michela_denti and Wouter Eilers and to @VArechavala and @AON_Delivery for pushing the publication of “negative results”. Unexpected or unhoped for results also advance science!
• • •
Missing some Tweet in this thread? You can try to
force a refresh




