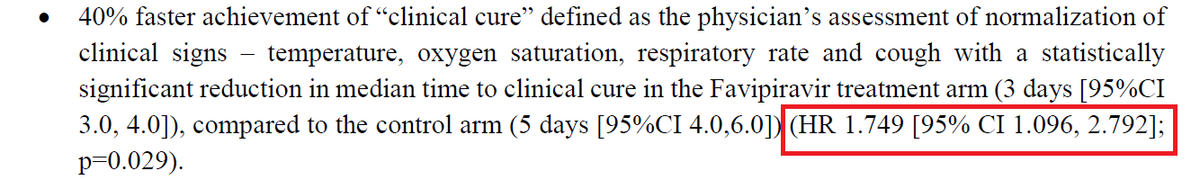1/n
The COLCORONA clinical trial (Lancet 27 May 2021) concludes that if we treat 70 Covid positive people with colchicine for a month, we prevent 1 hospital admission or death.
How did the researchers estimate the sample size of their study?
The COLCORONA clinical trial (Lancet 27 May 2021) concludes that if we treat 70 Covid positive people with colchicine for a month, we prevent 1 hospital admission or death.
How did the researchers estimate the sample size of their study?

2/n
Assumptions. They assumed that 7% of those on placebo shall either die or need hospital admission. Oral colchicine shall reduce this number to 5.25.
To detect a difference between the 2 arms of the study, they needed to enrol about 6000 people in the study. Which they did.
Assumptions. They assumed that 7% of those on placebo shall either die or need hospital admission. Oral colchicine shall reduce this number to 5.25.
To detect a difference between the 2 arms of the study, they needed to enrol about 6000 people in the study. Which they did.
3/n
The outcome in the colchicine trial was death or hospital admission. The secondary outcome was the need for mechanical ventilation.
Outcomes that are clinically meaningful. Outcomes that matter most to patients or their family.
The outcome in the colchicine trial was death or hospital admission. The secondary outcome was the need for mechanical ventilation.
Outcomes that are clinically meaningful. Outcomes that matter most to patients or their family.
4/n
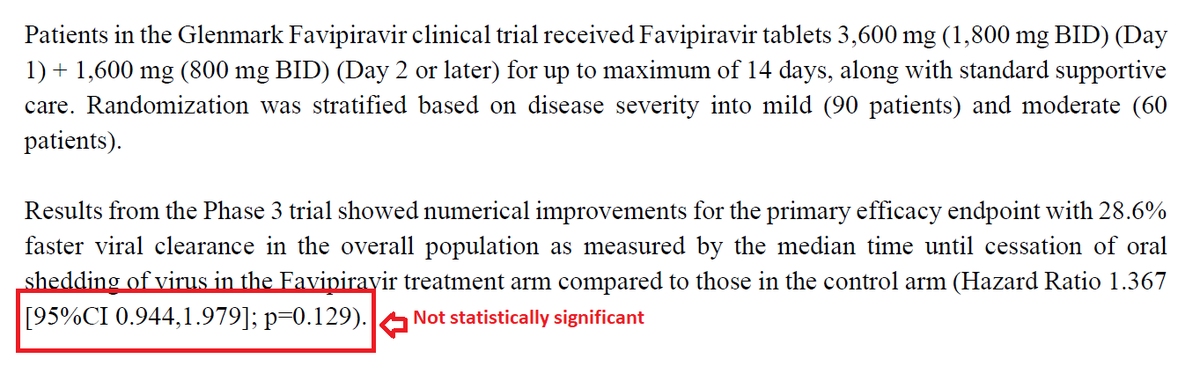
By contrast, let us look at the clinical trial that tested the efficacy of Favipiravir in mild Covid illness and made this drug so popular that it rocketed to the rank of the largest-selling pharma brand in India in April 2021.
By contrast, let us look at the clinical trial that tested the efficacy of Favipiravir in mild Covid illness and made this drug so popular that it rocketed to the rank of the largest-selling pharma brand in India in April 2021.

5/n
How many patients did favipiravir trialists enrol?
They enrolled 90 mildly ill and 60 moderately ill covid patients. Total, 150 patients. Yes. 150.
What outcome did they count? Viral clearance
What secondary outcome did they measure? Fever and cough.
How many patients did favipiravir trialists enrol?
They enrolled 90 mildly ill and 60 moderately ill covid patients. Total, 150 patients. Yes. 150.
What outcome did they count? Viral clearance
What secondary outcome did they measure? Fever and cough.
6/n
Researchers often term such outcomes as surrogate outcomes because they are not clinically meaningful.
In the Colchicine study, the researchers wanted to reduce death or hospitalisation.
Researchers often term such outcomes as surrogate outcomes because they are not clinically meaningful.
In the Colchicine study, the researchers wanted to reduce death or hospitalisation.
7/n
In the favipiravir study, the researchers were counting the cough and measuring the temperature or looking at viral clearance.
Even patients shrug off such outcomes with “Who cares” and “So what” gestures
In the favipiravir study, the researchers were counting the cough and measuring the temperature or looking at viral clearance.
Even patients shrug off such outcomes with “Who cares” and “So what” gestures
8/end
We need large-sized, multi-centric studies that ask a focused question, identify the most meaningful outcomes and set out to enrol thousands of patients to answer the question.
The contrast between Colchicine and Favipiravir could not have been more striking!
We need large-sized, multi-centric studies that ask a focused question, identify the most meaningful outcomes and set out to enrol thousands of patients to answer the question.
The contrast between Colchicine and Favipiravir could not have been more striking!
• • •
Missing some Tweet in this thread? You can try to
force a refresh