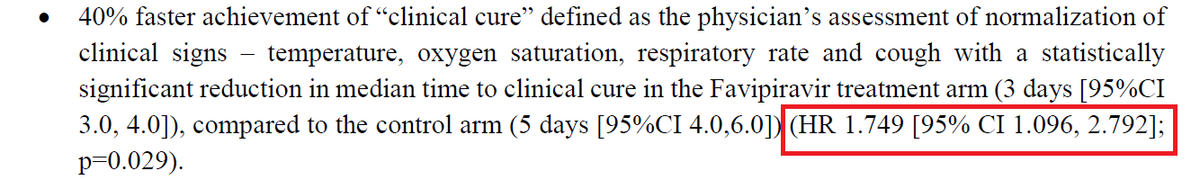I couldn't believe my eyes. So I asked my colleague to read to me the recent Covid19 guidelines by DGHS @MoHFW_INDIA. I couldn't believe my ears too.
The 9-page-PDF guides how to treat, investigate and monitor patients with Covid19 infection.
What is the first surprise? 1/N
The 9-page-PDF guides how to treat, investigate and monitor patients with Covid19 infection.
What is the first surprise? 1/N
Surprise 1.
Asymptomatic patients: "No investigation at this stage. And “No medications are required.”
No blood tests or fancy drugs for early Covid! 2/N
Asymptomatic patients: "No investigation at this stage. And “No medications are required.”
No blood tests or fancy drugs for early Covid! 2/N
Surprise 2.
Mildly ill patients: May need investigations if symptoms persist or they deteriorate.
Paracetamol and cough syrup. Budesonide.
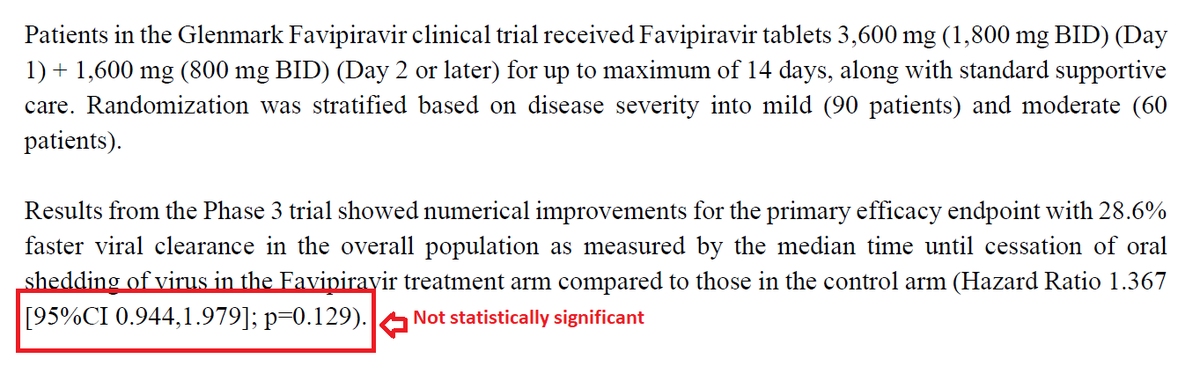
No other medicine required- So, no HCQ, Favipiravir, Ivermectin, Azithromycin, Doxycycline, Zinc and Vitamins. No plasma, either. 3/N
Mildly ill patients: May need investigations if symptoms persist or they deteriorate.
Paracetamol and cough syrup. Budesonide.
No other medicine required- So, no HCQ, Favipiravir, Ivermectin, Azithromycin, Doxycycline, Zinc and Vitamins. No plasma, either. 3/N
Surprise 3.
Moderately ill: Oxygen, control of co-morbidities, steroids, anticoagulants. Period. No coronil nor 2- DG.
4/N
Moderately ill: Oxygen, control of co-morbidities, steroids, anticoagulants. Period. No coronil nor 2- DG.
4/N
Surprise 4.
Severely ill: oxygen, steroid, anticoagulants and clear indications for Tocilizumab. Clear criteria for prescribing.
Need for recruiting the hospital infection control committee. 5/N
Severely ill: oxygen, steroid, anticoagulants and clear indications for Tocilizumab. Clear criteria for prescribing.
Need for recruiting the hospital infection control committee. 5/N
Surprise 5.
Diagnostic tests specifically mentioned. No Ferritin and LDH after day 1.
Clear indications for a chest x-ray.
Irrational and rampant use of HRCT Chest heavily criticised.
7/N
Diagnostic tests specifically mentioned. No Ferritin and LDH after day 1.
Clear indications for a chest x-ray.
Irrational and rampant use of HRCT Chest heavily criticised.
7/N
“Exercise extreme caution when ordering an HRCT.”
Full page on HRCT. When HRCT is needed; 4 bullet points on why routine HRCT shouldn’t be done and 4 bullet points on when HRCT should not be ordered!
8/N
Full page on HRCT. When HRCT is needed; 4 bullet points on why routine HRCT shouldn’t be done and 4 bullet points on when HRCT should not be ordered!
8/N
Surprise 6.
Full page on Remdesivir and Tocilizumab.
Advised to exercise extreme caution when ordering Remdesivir—“as this is only an experimental drug and has a potential to harm.”
Romance with remdesivir is over. This is unbelievable!
10/N
Full page on Remdesivir and Tocilizumab.
Advised to exercise extreme caution when ordering Remdesivir—“as this is only an experimental drug and has a potential to harm.”
Romance with remdesivir is over. This is unbelievable!
10/N
Surprise 7.
Full page on steroid and anti-coagulants.
Sensible, straightforward advice on steroid: Dexa— 6 mg a day, for 10- days, no taper, only in hypoxic patients.
Anticoagulants- prophylactic dose. Only in moderate or severely ill patients.
11/N
Full page on steroid and anti-coagulants.
Sensible, straightforward advice on steroid: Dexa— 6 mg a day, for 10- days, no taper, only in hypoxic patients.
Anticoagulants- prophylactic dose. Only in moderate or severely ill patients.
11/N
Surprise 8.
Full page on diagnosis, treatment and monitoring of Covid associated mucormycosis. The dose and duration of amphotericin B clearly spelt out. Clear guideline on when to switch to oral antifungal drugs.
12/N
Full page on diagnosis, treatment and monitoring of Covid associated mucormycosis. The dose and duration of amphotericin B clearly spelt out. Clear guideline on when to switch to oral antifungal drugs.
12/N
Today David Sackett, the father of EBM, would have been so happy!
Very impressive and the authors deserve high praise for sticking to EBM in the national guideline- conscientious, explicit & judicious use of current best evidence in making decisions for treating Covid19.
13/13
Very impressive and the authors deserve high praise for sticking to EBM in the national guideline- conscientious, explicit & judicious use of current best evidence in making decisions for treating Covid19.
13/13
• • •
Missing some Tweet in this thread? You can try to
force a refresh