
Excited to share our work on how antibodies are produced during SARS-CoV-2 infection! biorxiv.org/content/10.110… A brief summary... (1/n)
While protective antibody responses usually depend on T follicular helper (Tfh) cells, impaired Tfh responses have been observed in severe COVID-19: doi.org/10.1016/j.cell… We were therefore curious how this would affect antibody production to SARS-CoV-2. (2/n)
We infected mice lacking Tfh cells (Bcl6fl/fl;Cd4Cre) or all CD4+ T cells (Ciita-/-) with SARS-CoV-2. While most spike-specific IgG antibodies required CD4+ T cell help, substantial levels of these antibodies could be made in the absence of Tfh cells. (3/n) 

We found that “lymph node (LN)-Th1” cells express CD40L & IL-21 and co-localize with class-switched B cells. These are likely the non-Tfh CD4+ T cell population that promotes antibody production in the absence of, and perhaps in parallel with, Tfh cells. (4/n) 

LN-Th1-driven antibodies against spike and RBD were surprisingly high-affinity. Potential mechanisms include somatic hypermutation occurring outside of germinal centers and germline-encoded antibodies already being high-affinity, both of which have been reported. (5/n) 

Epitope profiling revealed that Tfh-dependent antibodies are strongly enriched for reactivity against S2 epitopes, such as those spanning fusion peptide. This suggests that the immunodominance of S2 epitopes seen in many human studies is Tfh-dependent. (6/n) 

However, both Tfh-driven and LN-Th1-driven antibodies demonstrated similar neutralization potency against homologous SARS-CoV-2 (USA-WA1/2020) as well as the B.1.351 variant of concern. (7/n) 
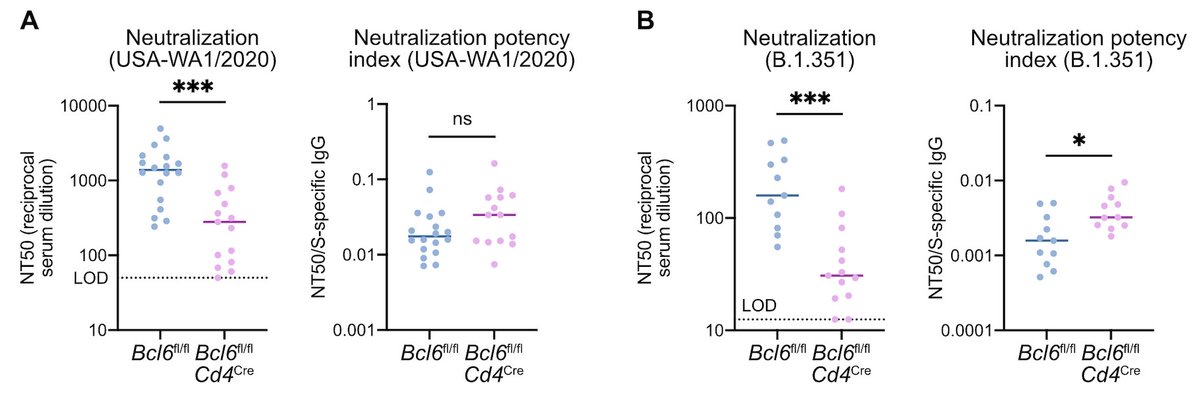
Therefore, we propose that Tfh cells and LN-Th1 cells promote complementary pathways of antibody production during viral infection to generate effective humoral immunity. (8/n) 

Extremely grateful for my amazing mentors @StephanieEisen and @WilenLab, wonderful collaborators @ScienceChow, @ericsongg, @tianyangmao, @BenIsraelow, Kathy Kamath, Joel Bozekowski, @hayneswa, Renata Filler, @biobridget, @JinWei0911, @MiaAlfajaro, Wenzhi Song, Lei Peng, (9/n)
Lauren Carter, Jason Weinstein, @UGowthaman83, @sidichen, Joe Craft, John Shon, @VirusesImmunity, and finally #fastgrants funding for making the whole project possible! (end)
• • •
Missing some Tweet in this thread? You can try to
force a refresh



