The #TTM2trial was published today in the @NEJM
Here’s a summary of what we did, what I think the trial means, and what next.
@ttm2trial @nielsen_niklas @CritCareReviews @NEJMnejm.org/doi/full/10.10…
Here’s a summary of what we did, what I think the trial means, and what next.
@ttm2trial @nielsen_niklas @CritCareReviews @NEJMnejm.org/doi/full/10.10…
We enrolled 1900 adults with a coma after an out of hospital cardiac arrest with a presumed cardiac or unknown cause
We assigned them to hypothermia at 33°C followed by controlled rewarming OR to normothermia with early treatment of fever (body temp ≥37.8°C)
The primary end point was death from any cause at six months.
Secondary outcomes included functional outcome at 6 months as assessed with the modified Rankin scale
In the 33°C arm we aimed for “gold standard” therapeutic hypothermia with cooling initiated early, slow rewarming, and care taken to avoid rebound fever.
In the early treatment of fever arm, fewer than half of the patients were cooled with a cooling device.
There was substantial separation in temperature by treatment group and the hypothermia arm was likely as good as can be achieved in clinical practice 

Some of the patients in the normothermia group developed fever and the temperatures in this group were broadly similar to those recorded in the control group of the pivotal Hypothermia after Cardiac Arrest trial in which no temperature management was used.
nejm.org/doi/full/10.10…
nejm.org/doi/full/10.10…
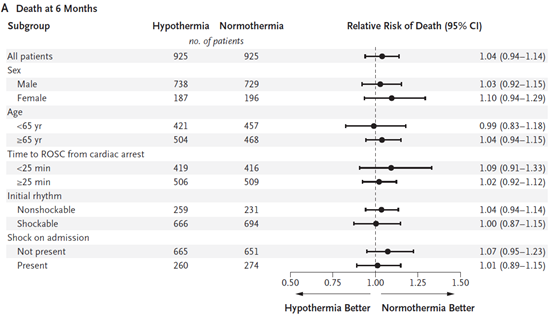
Despite this, at 6 months, 50% in the hypothermia group and 48% in the normothermia group had died (relative risk with hypothermia, 1.04; 95% confidence interval [CI], 0.94 to 1.14; P=0.37).
At 6 months, a total of 54% in the hypothermia group and 54% in the normothermia group had a poor functional outcome (relative risk in the hypothermia group, 1.00; 95% CI, 0.91 to 1.08).
Pre-specified subgroups were defined according to sex, age, initial cardiac rhythm, time to return of spontaneous circulation, and presence or absence of shock on admission.
Therapeutic hypothermia was associated with a significantly higher risk of arrhythmia resulting in hemodynamic compromise.
The @TTM2trial has many strengths including a sample size that was five times the combined enrolment of the earlier “positive” trials, centralised assessment of outcomes by trained assessors, multimodal neuroprognostication, and protocolisation of withdrawal.
Here are my take home clinical points…
(1) Therapeutic hypothermia is associated with a higher risk of arrhythmia and does not appear to benefit any patient group. The @TTM2trial data should herald the end of the era of therapeutic hypothermia for adult cardiac arrest.
(2) Core temperature should be monitored and current standard of care could reasonably be considered to be normothermia and early treatment of fever (body temp ≥37.8°C); however, here are a few things to think about…
Is normothermia better than no temperature control at all? The reality is that the evidence supporting normothermia over no temperature control is, at this point, very weak.
Given that fewer than half of the patients needed active cooling, do patients actually need to be sedated for a fixed period?
Why not just desedate patients who do not require cooling to avoid fever? This might mean patients who will do well can avoid unnecessarily prolonged ventilation. We need to study this.
Prior cardiac arrest trials that suggested benefit from therapeutic hypothermia were small and they were less methodologically rigorous than the @TTM2trial – all evidence is not equal and the @TTM2trial is the trump card.
For more discussion and to watch the results presentation check out criticalcarereviews.com
@threadreaderapp unroll
• • •
Missing some Tweet in this thread? You can try to
force a refresh