
It's #ReadAlong Day!
@UCSB_OakleyLab will #TakeOver our account at 12 pm ET.
Follow this thread for live tweets as we #ReadAlong: Warner & Case, 1980 (journals.uchicago.edu/doi/pdf/10.230…)
Drop your questions and comments in this thread.
Happy Reading! 🧵(1/n)
@UCSB_OakleyLab will #TakeOver our account at 12 pm ET.
Follow this thread for live tweets as we #ReadAlong: Warner & Case, 1980 (journals.uchicago.edu/doi/pdf/10.230…)
Drop your questions and comments in this thread.
Happy Reading! 🧵(1/n)
https://twitter.com/BiolBulletin/status/1427456290507444224
G’Day! I am thrilled to have the opportunity to lead today’s #ReadAlong with Biological Bulletin!
This is Todd Oakley @ucsb_oakleylab. I am a Professor at the University of California, Santa Barbara @eembucsb. (2/n)
This is Todd Oakley @ucsb_oakleylab. I am a Professor at the University of California, Santa Barbara @eembucsb. (2/n)
My research focuses on how complexity originates during evolution, which has led me to mainly study the origins of eyes/vision and of bioluminescence in animals. (3/n)
Since most animal diversity is invertebrate and much of that is marine - our lab has studied quite a few different marine invertebrates. (4/n)
I’ve been on the Editorial Board @BiolBulletin for a few years now and I am continually impressed by the enthusiasm and energy of the EiC Ken Halanych, by the Staff (Carol and @crustyolfactory), and all of the Editors. (5/n)
The Biological Bulletin embodies for me several things that I love most about science. First, is a sense of wonder for the natural world. The journal is a venue for scientists to communicate scientific findings inspired by their curiosity about nature. (6/n)
Second, science brings together like- minded people, such as the fantastic group at the journal. But perhaps my favorite thing about science is the connection across time to people, traditions, and ideas from the past, while using new tools and insights from the present. (7/n)
Biological Bulletin has been a journal for 125 years, recording the passions, curiosities, and results of scientists all along. I think these #ReadAlong series highlight exactly this connection between past and present. (8/n)
I have chosen a classic paper, from 1980 - and we will communicate about it in real time, allowing scientists anywhere in the world to join in. In 1980, this would have been almost unimaginable science fiction! (9/n)
I chose a paper written by Jon Warner and Jim Case who were both at @ucsantabarbara when they wrote this one. I overlapped with Jim Case when I first started at UCSB and we even have several pieces of equipment we inherited from him to this day! (10/n)
I chose this paper in part because of the @ucsantabarbara connection, but also because one of the characters of the story is a bioluminescent ostracod, which are some of my favorite critters! (11/n)
Spoiler alert -- this paper establishes that a bioluminescent fish -- Porichthys notatus -- must eat bioluminescent ostracods (Vargula tsujii) before the fish itself can bioluminesce! (12/n)
One reason this is interesting is that these ostracods use bioluminescence as an anti-predator display - so it's surprising that Porichthys must eat ostracods. Here is an example of a cardinalfish trying to eat a luminous ostracod 👇(13/n)
Our lab studies the bioluminescent ostracods, or “sea fireflies” -- as well as the fish Porichthys. Our long term goal is to understand how evolution shapes the genes involved in creating and using the light they use for bioluminescence. (14/n)
Besides the anti-predator signal, another use of bioluminescence for the seafireflies is courtship. Here is an example of a display by Maristella chicoi in Belize 👇(15/n)
We even keep Vargula tsujii in the lab now! Here is a paper led by @sluglife28 on how we figured this out: nature.com/articles/s4159…
(16/n)
(16/n)
But what is the fish using its bioluminescence for? The fish has light producing organs all along its belly called photophores. These light up to match light from above so the fish doesn’t cast a shadow below, in a process called counter-illumination. (17/n)
Here is an old video from Jim Case’s lab showing Porichthys counter-illuminating! I think @beroe was present for this and might have more to share about it (18/n)
With that backdrop, let’s dive into this paper on dietary induction of bioluminescence in a fish! (19/n)
Usually when I read a paper for the first time, I jump around in my reading a lot. Something like, Title, Abstract, Intro, Discussion, Results, Methods; all the while looking at figures too. In our lab group discussions of papers, we go through figures to guide discussion. (20/n)
But for this #ReadAlong I think I will go straight through from beginning to end. The introduction is 6 paragraphs, let’s take those one at a time. (21/n)
IP1 sets the stage, telling us that scientists already know there is chemical cross-reactivity between the bioluminescence systems of the star of the paper, the midshipman fish, and that of ostracods like Vargula hilgendorfii. (22/n)
V. hilgendorfii lives in Japan - not in California where P. notatus lives. But V. hilgendorfii has always served as a sort of model system for bioluminescent ostracods. (23/n)
Also in Intro Paragraph 1 (IP1) the authors mention Parapriacanthus a fish from Japan. This fish led to some amazing science, published only recently. Manabu Bessho-Uehara (@Parapriacanthus) & colleagues discovered a new biological phenomenon they called... (24/n)
... “kleptoprotein bioluminescence.” Basically, the fish eats a luminous ostracod to steal its luciferase protein to make light! advances.sciencemag.org/content/6/2/ea… (25/n)
So the authors suspected the California midshipman fish might eat ostracods to gain bioluminescence, but could it be other animals instead or in addition? In IP2, they mention a then-new ostracod to the science-scene: Vargula tsujii. (26/n)
The fish, P. notatus lives from Baja CA to Alaska, but no one knew of any luminous ostracods in that range until 1977 when Kornicker and Baker described Vargula tsujii... (27/n)
Maybe that is the source of fish bioluminescence then!!
(Also a quick mention of my science heroes, Lou Kornicker who lived to be 100 years old and described most of the myodocopid ostracods!). (28/n)
(Also a quick mention of my science heroes, Lou Kornicker who lived to be 100 years old and described most of the myodocopid ostracods!). (28/n)
In IP3 the authors summarize some of the biology of the fish P. notatus. They mention the photophores all along the belly that house the light reaction and the structures that house the substrate “luciferin” of the biochemical reaction. (29/n)
The next paragraph, IP4, reveals an interesting twist about the biology of P. notatus that was another clue to how they get the luciferin substrate. Fish that live in the south are bioluminescent, but fish that live up north from the same species are non-luminous! (30/n)
In previous work, scientists including bioluminescence-expert Fred Tsuji (for whom our ostracod in named) injected luciferin in the northern population and they gained the ability to bioluminesce!! (31/n)
In IP5 the authors point out that they can use fluorescence to check fish for the luciferin substrate that allows them to produce bioluminescence. Unlike much of the recent findings on biofluorescence (attn: @mikebok) - this result is linked to a function. (32/n)
Southern fish glowed and northern ones didn’t, correlating with their ability to make light. Here is a photo from @chipotlau showing this fluorescence:
https://twitter.com/chipotlau/status/1099138021348134912?s=20(33/n)
And the last paragraph of the Introduction is a brief summary of the main result: When the fish eats V. tsujii the fish can bioluminesce!! Not only that, the limited distribution of V. tsujii in the south explains why fish there light up, but northern fish do not!! (34/n)
On to the Materials and Methods! The Methods are highly integrative and interdisciplinary, like those of many papers in @BiolBulletin. At the same time, the methods in this paper are pretty straightforward and divided into 5 sections. (35/n)
Methods section 1 (MS1) Field Studies. They sampled 9 sites around California using bottom trawls from 5-500 m depth to catch P. notatus. The fish live near the bottom at these depths for most of the year, but they do spawn in the intertidal during summer. (36/n) 

On board the ship, the authors looked for fluorescence to indicate luciferin, and also they tested for bioluminescence by injection. (37/n)
Today, we get our fish at UCSB from fishing boats who get the midshipman in by-catch.
MS2 - Laboratory Studies. In the lab, they also measured bioluminescence, again after injecting DL-arterenol (a hormone). (38/n)
MS2 - Laboratory Studies. In the lab, they also measured bioluminescence, again after injecting DL-arterenol (a hormone). (38/n)
To measure the light, they used a photomultiplier tube (a PMT), which is an instrument that can measure dim light, even a single photon! (39/n) 

Check out the great figure (Figure 2) showing their PMT setup! Can anyone read the initials in the bottom right corner? I wonder who made this fantastic drawing. Pen and ink original, it looks like! (40/n) 

MS3 - Feeding experiments. So they suspected that V. tsujii is the dietary source of light, but it could be other organisms also. For some, the chemistry was unknown so other luminous animals might have the same light-chemistry as the ostracods. (41/n)
Therefore, they fed a bunch of different luminous animals to the fish, to see if any would allow bioluminescence ability - listed in Table 2. (42/n) 
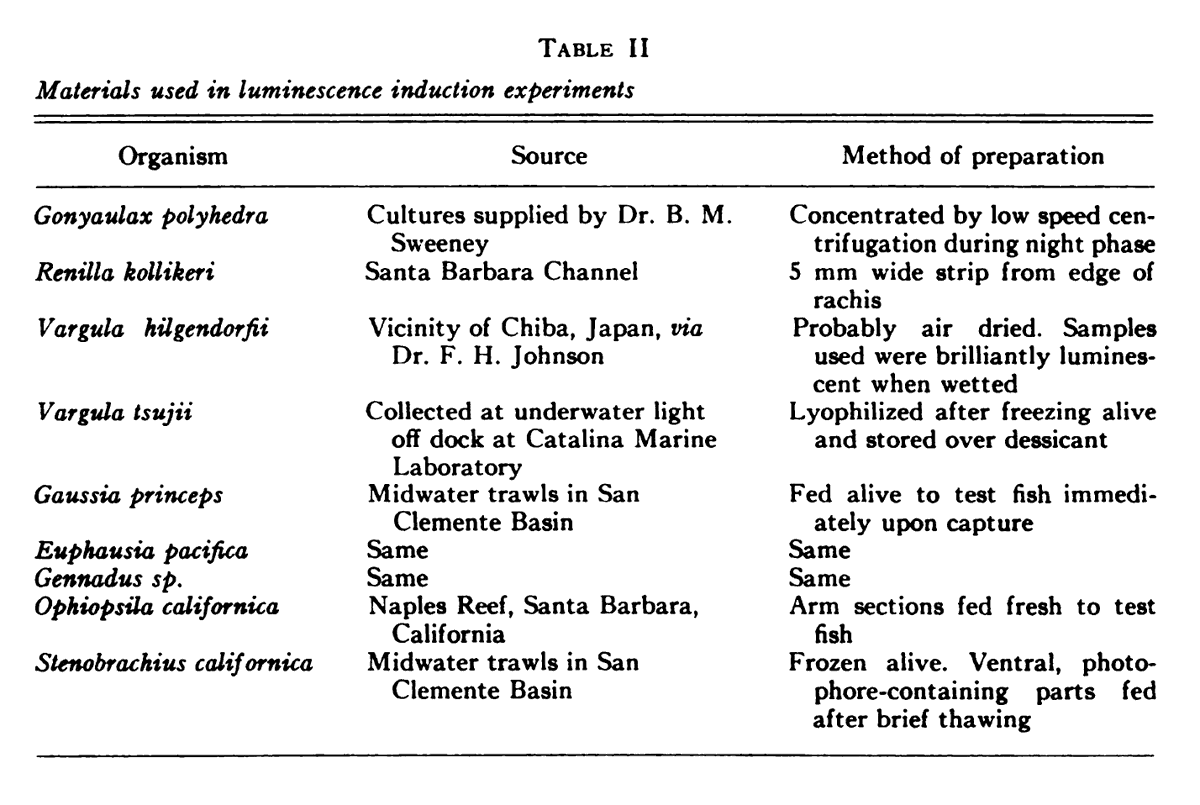
MS4 - Studies on fluorescence induction. They fed a bunch of ostracods (the Japanese model, V. hilgendorfii) to a non-luminous P. notatus to see how long it took before its photophores would start to fluoresce, as an indicator of luciferin from the ostracod getting there. (43/n)
MS5 - Behavioral Studies. And in the final methods section, they described their behavioral set up. Basically, they induced bioluminescence in various ways and measured the light from below with a PMT. (44/n)
I am guessing this is basically the same setup used for the video from Case lab I shared earlier.
That wraps up the Methods -- on to the Results! (45/n)
That wraps up the Methods -- on to the Results! (45/n)
There are 5 sections to the Results -- these are similar to the 5 Methods sections, but a bit different... (46/n)
Results Section 1 (RS1) refers to Figure 1. There is a curious and conspicuous gap in the distribution of P. notatus. Basically, they don’t seem to like Oregon! (47/n) 

I don’t know much about biogeography -- maybe @wareslab has some thoughts on this. I thought Point Conception was the usual break in the California Pacific! (48/n)
RS2 Correl. of fluorescence with bioluminescence in natural populations. Sure enough! Every time they find fluorescence of the photophores of the fish, those fish are able to bioluminesce (& non-fluorescent don’t light up)! The results are summarized with symbols on Fig 1. (49/n)
This fluorescence is really beautiful. Here is another photo, from Darrin Schultz (@conchoecia) under fluorescent light. Every dot is a photophore! These photophores are naturally silvery, reflecting the bioluminescent light away from the fish. (50/n) 

This is how midshipman-fish get their name. The photophores look like silver buttons on a Naval officer’s coat! (51/n)
RS3 are the results of the fluorescence induction. As shown in Figure 3, it takes about 3 days from the time the fish eats ostracods until the photophores luminesce. A nice clean, clear result! (52/n) 

RS4 is one of the central results - feeding Vargula tsujii to Porichthys notatus allows the fish to start bioluminescing. Not only that, other luminous species from California fail to allow bioluminescence when fed to the fish. (53/n)
I like how they made sure those fish *could* bioluminesce by feeding ostracods after the initial test with the other species. (54/n)
RS5 is the behavioral result. Sure, it’s great that there is fluorescence and bioluminescence after feeding ostracods to the fish. But can the fish actually USE this bioluminescence, or are we just creating an overly artificial situation in the lab? (55/n)
Well, it is not easy to see these fish bioluminesce in the wild, especially in 1980 -- but this is where the behavioral experiments came in. The fish actually did create the bioluminescence “on their own”, not only after injecting hormones (Table 4). (56/n) 

So this starts to suggest dietary acquisition in the wild is how the fish get their luciferin! (57/n)
That concludes the Results section -- we will conclude with the Discussion next! (58/n)
Let’s hit the home stretch and go through the Discussion section! Compared to the other sections, the Discussion is quite a bit longer. So instead of paragraph by paragraph, I will highlight a few major themes: (59/n)
1) Biogeographical distributions.
2) Recycling of the luciferin.
3) Evolutionary origins and maintenance of bioluminescence.
(60/n)
2) Recycling of the luciferin.
3) Evolutionary origins and maintenance of bioluminescence.
(60/n)
First off - some riffs on the distributions. The core result is that V. tsujii and P. notatus overlap in the South of the fish’s range, allowing bioluminescence there. But the overlap between luminous fish and V. tsujii is not perfect either. (61/n)
One question is Baja California. The authors were unable to get fish from that far south, so we don’t know if they are luminous there. There is a report of luminous V. tsujii there, so a hypothesis is that the fish are also luminous there. (62/n)
On the northern side, there is pretty good correspondence between the ranges of V. tsujii and luminous P. notatus -- both go up to about Monterey Bay in the literature. However, we have not had much success catching V. tsujii (in limited attempts). (63/n)
They are scavengers and come to traps. We (especially Lisa Mesrop) get them routinely from Catalina Island. But the farthest north we’ve found them is rarely here in Santa Barbara. (64/n)
The authors also mind the gap in the distribution of P. notatus. Why are they not in Oregon? They hypothesize that summer water temperatures are too cold for intertidal spawning. Any thoughts on that @wareslab? (65/n)
Another prominent topic in the Discussion is recycling of the luciferin substrate. Basically, once the fish get a little bit of luciferin, they can keep creating light for a LONNNGGG time, even without eating more ostracods. (66/n)
This is surprising because the luciferin is quite unstable. It oxidizes easily and would get used up or “spoiled”. So that leads to the hypothesis that the fish can recycle it after it is used for bioluminescence. (67/n)
Given new multi-omic tools that are available today, figuring out what is going on here would be a really fun project! (68/n)
So the last Discussion topic I will riff on is my favorite topic OF ALL TIME -- how do complex traits originate during evolution? (69/n)
The authors provide some fun thoughts on this, again mentioning the Japanese fishes Apogon and Parapriacanthus that I mentioned earlier. As a reminder, Parapriacanthus eats luminous ostracods and [checks notes] STEALS THEIR LUCIFERASE PROTEIN! (70/n)
To clarify, this kleptoprotein bioluminescence is not what is going on in P. notatus/V. tsujii. In fact, Parapriacanthus has a physical connection between its gut and the photophores (light-producing organs). (71/n)
This is not the case in P. notatus, so it seems it would be much more difficult to steal a protein. Also, P. notatus can gain bioluminescence by injecting the substrate alone, ruling out the kleptoprotein idea. (72/n)
So back to the origins idea -- how might it have come about that P. notatus relies on V. tsujii for luciferin? The authors kind of anticipate the amazing work of @parapriacanthus on kleptoproteins when they write... (73/n)
“the fish luminescent system [may have been] initially obtained in its entirety from the ostracod”.
YUP! That is what is going on in Parapriacanthus
advances.sciencemag.org/content/6/2/ea… (74/n)
YUP! That is what is going on in Parapriacanthus
advances.sciencemag.org/content/6/2/ea… (74/n)
But they dismiss their hypothesis because (as they state), “the fish would have obtained the biosynthetic elements of the luminescent system before developing the photophore and control mechanisms.” (75/n)
And yet, to me, the Parapriacanthus system seems to be a quite logical intermediate state. Use bioluminescence from a direct connection between gut and simple photophore as a precursor to the more gradual evolution of a disconnected, complex photophore. (76/n)
I think I will stop here. There are so many more interesting things that I can think to discuss relating to this paper and if ideas or questions come up, I am very happy to discuss those with you on my account @UCSB_OakleyLab. (77/n)
I will conclude by thanking @crustyolfactory -- the Wizard behind the curtain making me look more Twitter savvy than I really am… Thank you! Stay tuned here for future #ReadAlong events with @BiolBulletin! (Fin)
Please unroll @threadreaderapp
• • •
Missing some Tweet in this thread? You can try to
force a refresh






