
Finally the study we've all been waiting for on mix&match boosters.
Results: J&J starts at 6-15x lower antibodies than Pfizer/Moderna. It's boosted 56x by Moderna, 5x by another J&J.
Clearly Moderna is the better booster for #JnJers
All as predicted
medrxiv.org/content/10.110…
Results: J&J starts at 6-15x lower antibodies than Pfizer/Moderna. It's boosted 56x by Moderna, 5x by another J&J.
Clearly Moderna is the better booster for #JnJers
All as predicted
medrxiv.org/content/10.110…

Note J&J starting at 6x lower abs than Pfizer and then boosted 5x by a 2nd J&J dose means J&J x2 gets you to where Pfizer "fully immunized" people were to begin with. That pretty much proves what many of us have been saying: J&J should have gone for a 2-dose vax to begin with. 

Also a J&J booster is also not as effective in raising antibodies for Pfizer and Moderna recipients as a Moderna booster. 

Pfizer boosters are only halfway through testing (day 29 not available) so I didn't discuss earlier, but am now showing the Pfizer boost data below. The effects of Pfizer boosters are in between those of J&J and Moderna boosters. 
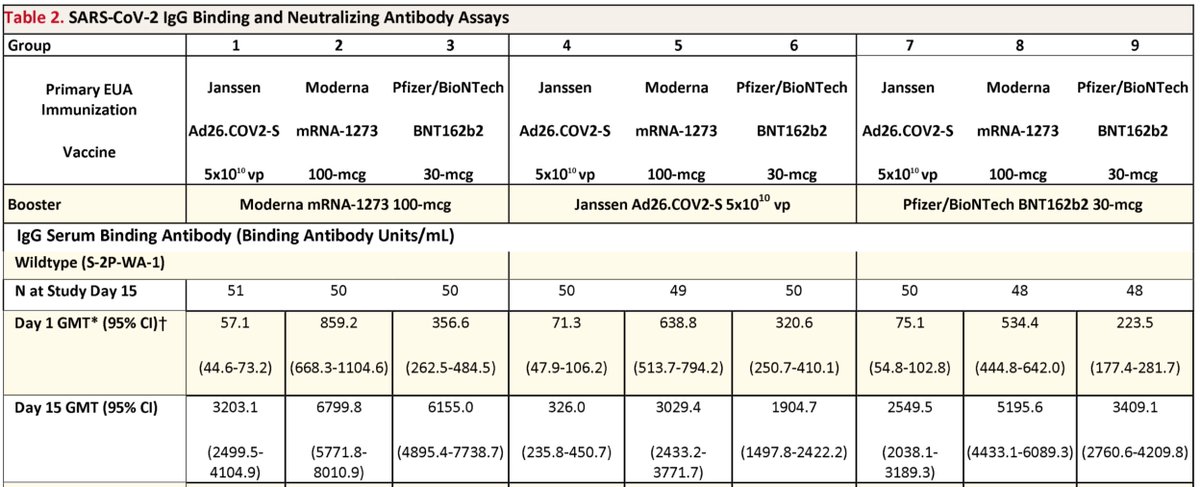
The Moderna vs J&J results are as expected, as we knew that per-shot immunogenicity (that is the ability of *one* shot to raise antibodies) is Moderna > Pfizer ~ J&J.
You can go back to the Phase 1-2 trials and it's all there: 1x J&J ~ 1x Pfizer, 2x J&J ~ 2x Pfizer in Ab levels
You can go back to the Phase 1-2 trials and it's all there: 1x J&J ~ 1x Pfizer, 2x J&J ~ 2x Pfizer in Ab levels
The one perhaps unexpected result is a Pfizer boost working better for Pfizer/Moderna than a J&J boost. Some expected that RNA+Ad would be better than RNA+RNA.
But I never understood that myself as it wasn't clear to me JJ's supposed superior T cell stim was useful for boosting
But I never understood that myself as it wasn't clear to me JJ's supposed superior T cell stim was useful for boosting
Actually, could be that J&J boost ends up working similarly to Pfizer boost. It's likely that Pfizer boost effects will peak earlier than J&J boost and then drop a little, like Moderna boost. That would indeed be consistent with per-shot immunogenicity of J&J ~ Pfizer, eventually 

Apologies for the many crops of the same table; was trying not to overwhelm at the beginning with too many numbers but eventually realized I had to show the whole table to predict how Pfizer boosts would turn out.
BTW the fold changes in the top tweet were for day 15 as that's what the authors calculated, but I circled day 29 titers in the graphic as that's more relevant.
Glad the authors included Pfizer rather than leaving it out entirely, or waiting another 2 weeks to post the results
Glad the authors included Pfizer rather than leaving it out entirely, or waiting another 2 weeks to post the results
Here are the Phase 1-2 serology data from summer 2020 showing immunogenicity of a single shot ranks as Moderna > Pfizer > J&J. These results showed 1 shot Moderna elicits ab levels ≥ mean convalescent sera, while 1 shot J&J or Pfizer gives ~50% of mean convalescent sera. 





I showed results for older adults as they need protection from infections the most, but younger adults showed the same trend (with higher abs in each case). You can look yourself at
nejm.org/doi/full/10.10…
nejm.org/doi/full/10.10…
nejm.org/doi/full/10.10…
nejm.org/doi/10.1056/NE…
nejm.org/doi/full/10.10…
nejm.org/doi/full/10.10…
nejm.org/doi/full/10.10…
nejm.org/doi/10.1056/NE…
J&J also tested 2 doses (in younger adults only), and saw it produced antibody levels higher than convalescent. Of course Pfizer also got higher ab levels after 2 doses.
So with similar results after just 1 dose, J&J chose to test that in Phase 3 while Pfizer tested 2 doses.

So with similar results after just 1 dose, J&J chose to test that in Phase 3 while Pfizer tested 2 doses.


So we can see how Pfizer and J&J single-dose immunogenicity is similar, but Pfizer (and Moderna) chose to test 2 doses as a complete vaccine while J&J opted for 1. FDA and CDC decided to conflate them as "fully vaccinated" despite different performance, causing confusion since.
Typo, meant Moderna > Pfizer ~ J&J although you could also claim Moderna > Pfizer ≥ J&J from these data
And finally, why does J&J boost fall short of Pfizer boost for JnJers if, as I presented above, shot#1 of J&J is about as immunogenic as shot#1 of Pfizer (and J&J boost might trend to approach Pfizer boost for Pfizer/Moderna)?
Likely it's because antibodies elicited to the adenovirus capsid in JnJers partly neutralize later J&J doses, blunting the effects of that booster. This is not seen in RNA vaccines because they don't have protein components and (AFAIK) you don't get antibodies to the RNA itself.
As @JGPharmD has pointed out, Moderna filed an EUA for a 50mcg booster, in between the 100mcg Moderna and 30mcg Pfizer tested here. Moderna must have other data on 50mcg but for Moderna recipients not J&J. This presents opportunities for confusion at FDA and CDC meeting.
That is, FDA staff and advisors may find the Moderna at 100mcg here compelling enough to suggest amending the original Moderna EUA to cover its use as a booster for J&J, yet not for Moderna as Moderna has a separate EUA application for that already.
Or they could say let's not confuse people on Moderna and let's just amend the Pfizer EUA to allow its use as a booster for J&J. (Not possible to change Pfizer fully approved uses in a reasonable time, but CDC could separately allow off-label Pfizer use via the regular approval.)
But then some staff/advisors might insist on waiting another week for Pfizer 29-day results from this study, and it would be strange to amend Pfizer EUA and not Moderna EUA for JnJers if Moderna EUA is as good or better.
Worst-case scenario: FDA accepts J&Js application to amend *their* EUA because they applied and it doesn't raise these questions of how to treat Pfizer and Moderna fairly relative to each other, even though the J&J booster results are clearly the worst.
What do I think should happen? FDA should allow Moderna and Pfizer boosts for JnJers. But if they don't CDC should state that Pfizer can be used to boost JnJers via off-label Rx, bypassing the need for FDA action. CDC can ask ACIP if they want, or Walensky can just do it.
One more thing: only the J&J x2 regimen failed to elicit neutralizing Abs above the level needed for 90% protection from infection (pre-Delta). I marked this level on the image below.
That gives an idea of what the boosters do for J&J:
J&J+ J&J: <90% VE
J&J + others: >90% VE
That gives an idea of what the boosters do for J&J:
J&J+ J&J: <90% VE
J&J + others: >90% VE

I don't usually cite neutralizing antibody levels in absolute terms because different nAb assay protocols give very different results, so you can't always compare between studies. I actually find this very interesting as it relates to our my lab's own work so I'll explain a bit.
In a neutralizing antibody assay you put virus on cells in the presence of different amounts of serum and see what amount is required to block the entry. The more serum you need, the lower the nAb titer.
Sounds simple enough, but each of those steps can be done a different way. You can use real SARSCoV2 virus but that requires BSL3, or you can use a different virus (usually lentivirus but sometimes VSV) made to express SARSCoV2 spike protein on its surface, and do it at BSL2.
This kind of substitute virus is called a pseudovirus, but it's a real virus, just not SARSCoV2. An advantage of the pseudovirus is you can quickly try various SARSCOV2 spike mutants; this isolates evasive effects of spike mutations out of all genetic differences between strains.
Another advantage is you can put a reporter gene such as a light-emitting luciferase into the pseudovirus genome. This makes assaying how much blockade of infection you get easier; you just assay light emission as it should be proportional to the amount of virus that gets in.
The last condition that can vary is the cell line infected. As spike mediates attachment to ACE2, you can use any line that expresses ACE2. This includes HEK293T cells, which are easy to grow and introduce genes into and one of our favorite cell lines, made to express ACE2.
The nAb assay used in yesterday's paper was lentivirus expressing SARSCoV2 spike with D614G mutation and luciferase on HEK293T-ACE2 cells. All that jargon is needed to verify it's the same assay as in the previous study correlating 100 IU50/mL with 91% VE.
medrxiv.org/content/10.110…
medrxiv.org/content/10.110…
Actually the previous study didn't describe the assay, but referred to 3rd paper which did (below). And it does appear to be the same assay.
ncbi.nlm.nih.gov/pmc/articles/P…
ncbi.nlm.nih.gov/pmc/articles/P…
So there's still the potential for inter-lab differences in how the assay is run but at least you can now interpret the numbers obtained here using the clinical correlations established in the previous paper.
(I find the assay interesting because it uses very common parts: A biologist who has worked with lentivirus, luciferase, and HEK293T cells, which is probably the majority of cell biologists, can set up the assay using cells and genes already in the lab)
So now that we have verified that 100 IU50/mL is indeed a meaningful nAb level that may correlate with 90% VE against pre-Delta strains, and that only the J&J + J&J regimen fails to get over that level, we know the implications of what VRBPAC are being asked to evaluate tomorrow.
VRBPAC is being asked to evaluate J&J's request for booster approval either at 2months (citing ENSEMBLE2 results with serology and clinical data) or at 6mo (citing <20 patients with serology only).
The J&J boost at 2mo in the ENSEMBLE2 trial raised antibodies by ~5 fold and did not lead to a discernable increase in protection against disease (estimated pre-Delta VE was 67% for 1 dose vs. 76% for 2, but confidence intervals are mostly overlapping)
https://twitter.com/michaelzlin/status/1448415709801811975
This makes sense from what we just discussed above in the NIH study: J&J + J&J boost at 3-4 months caused a 5x increase in antibodies (similar to the ENSEMBLE2 findings) but this is below the level needed for 91% preDelta VE. So an early boost of J&J by J&J is not great.
But VRBPAC could still support approval for J&J boosters after 6mo based on the 12x increase in antibody levels. That's still not as good as the 56x or 33x boost by Moderna or Pfizer but it's better. Problem is the 12x came from a tiny study of <20 people
https://twitter.com/michaelzlin/status/1440401798397714436
I think it's unlikely VRBPAC will make any recommendations for a Moderna or Pfizer booster, although these are clearly better based on yesterday's NIH study. It's because FDA is asking VRBPAC to comment on J&J's application, not on this independent study.
It will be interesting to see if FDA, after VRBPAC has commented on J&J's application, will amend the EUAs for Moderna and Pfizer to allow their use as boosters for J&J, regardless of what is decided for J&J. FDA can do that; the VRBPAC is advisory only.
CDC could also just state the Pfizer can be used as a booster off-label from its fully approved indication, which is what UK allows.
Or CDC and FDA could wait another 2 weeks for the next VRBPAC meeting to decide on allowing heterologous boosters for JnJers... 🐢
Or CDC and FDA could wait another 2 weeks for the next VRBPAC meeting to decide on allowing heterologous boosters for JnJers... 🐢
• • •
Missing some Tweet in this thread? You can try to
force a refresh






