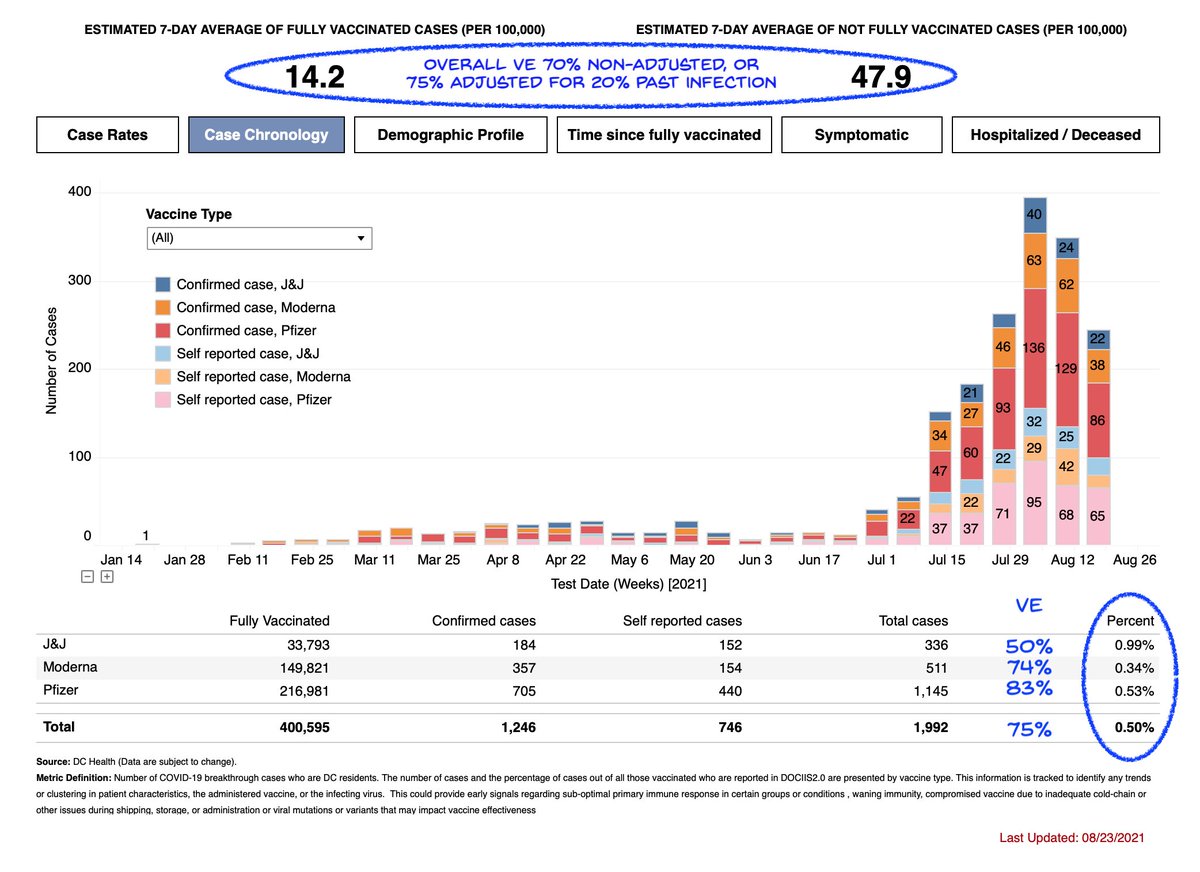
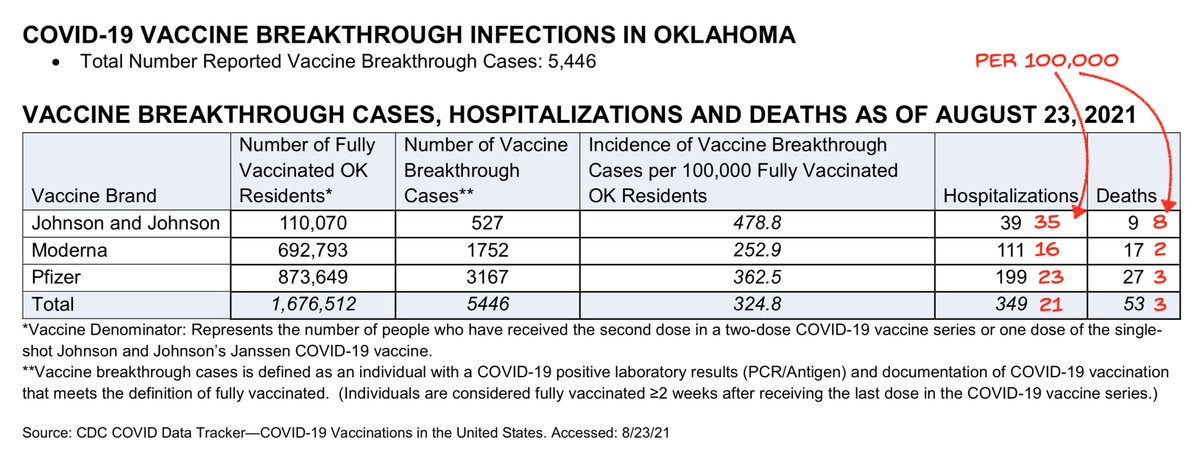
The VRBPAC meeting shows how FDA okays *indications*: a drug must prove efficacy for a defined health condition. But this is defined for individual patients. For vaccines the fxn of stopping infection chains is ignored.
Thus FDA is ill suited for the booster question.
Thus FDA is ill suited for the booster question.
CDC could have solved this problem by simply issuing guidance about who can get a Pfizer booster via the off-label pathway.
Sure, if they wanted, they could ask ACIP first. If CDC were to present the right data, ACIP would approve.
But asking FDA means PH was not considered.
Sure, if they wanted, they could ask ACIP first. If CDC were to present the right data, ACIP would approve.
But asking FDA means PH was not considered.
Put another way FDA and CDC may demand different burdens of proof due to their different missions, with FDA requiring incontrovertible evidence of efficacy and safety (unless you're Biogen), while CDC should promote anything easy that can promote public health.
Thus it's too bad the WH didn't anticipate FDA advisors and staffers applying SOP to limit boosters. Either they should have asked FDA to formulate PH criteria in this special case in a deviation from SOP, or they should have gone with a CDC-driven offlabel Pfizer boost strategy.
Finally it makes you realize how damn lucky we were that Pfizer and Moderna performed their trials so quickly and actually met all FDA efficacy, safety, manufacturing and documentation requirements before the first epidemic winter.
We may not be so lucky with the next epidemic, so it would be good to create FDA public health emergency evaluation procedures to use for new vaccines and treatments that are needed urgently and have non-autonomous benefits.
One speed and one SOP does not fit all situations
One speed and one SOP does not fit all situations
An obvious solution: a joint FDA-CDC task force for vaccines, another for treatments, each small for rapid discussion and decision-making, but able to demand data collection and statistical analysis from FDA or CDC staff if desired.
In stories such as below from @politico, you can see how the different takes on vaccines from FDA and CDC officials, stemming from their traditionally distinct health missions, has confused press reports on boosters.
politico.com/news/2021/09/1…
politico.com/news/2021/09/1…
Americans are confused, but haven't realized the confusion derives from a FDA-CDC mission gap.
Critical for HHS secretary or POTUS to realize this, describe the problem to the public, and propose how to fix it with a combined mission definition that coordinates left+right hands
Critical for HHS secretary or POTUS to realize this, describe the problem to the public, and propose how to fix it with a combined mission definition that coordinates left+right hands
Our current approach is like launching D-Day by letting army and navy formulate and carry out plans independently, with veto power over the other's plan. Result is paralysis.
Leadership needs to ID the problem and force agencies to work together in a task force (not committees).
Leadership needs to ID the problem and force agencies to work together in a task force (not committees).
• • •
Missing some Tweet in this thread? You can try to
force a refresh