FDA panel greenlighted Moderna booster vaccines. CDC will consider it this week. My thoughts 🧵:
🔵 Moderna booster definitely enhances immunity
🔵 Moderna boosters for people > 65 at 6+ months is reasonable, and healthcare workers
🔵 Moderna booster needed less than Pfizer
🔵 Moderna booster definitely enhances immunity
🔵 Moderna boosters for people > 65 at 6+ months is reasonable, and healthcare workers
🔵 Moderna booster needed less than Pfizer
🔵 The Moderna booster definitely enhances immunity
Over the Moderna booster data and FDA filing look good. The data are more limited than desired, but it all makes sense.
Over the Moderna booster data and FDA filing look good. The data are more limited than desired, but it all makes sense.

The FDA probably struggled with the idea of making a Moderna booster recommendation different than the Pfizer booster recommendation they recently made.
Reducing the Moderna dose created a regulatory challenge, as Moderna has to justify the changed dose. It is reasonable for the FDA to not be thrilled about the limited data supporting that change. Nevertheless, I think thumbs-up was the right decision.
The half-dose booster (50mcg) clearly gives a big antibody boost, and it is probably less reactogenic (side effects) than a higher dose.
The booster antibody responses look good, including enhanced neutralizing antibody breadth against variants.
The data reviewed by the FDA were primarily the Moderna booster data first reported in May, and recently published in Nature Medicine.
nature.com/articles/s4159…
The data reviewed by the FDA were primarily the Moderna booster data first reported in May, and recently published in Nature Medicine.
nature.com/articles/s4159…
Given the booster immunogenicity, + concerns about waning immunity, recommending Moderna boost for people > 65, and healthcare workers, at 6+ months is reasonable.
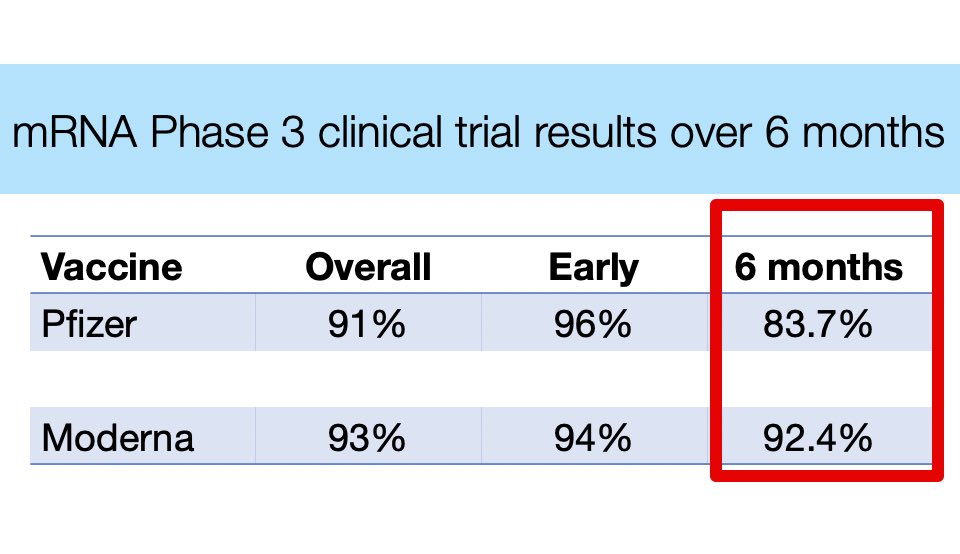
But 1) Moderna looks more protective than Pfizer, 2) Waning immunity is still debated, particularly for Moderna.
But 1) Moderna looks more protective than Pfizer, 2) Waning immunity is still debated, particularly for Moderna.
1/ Moderna looks to be somewhat more protective than Pfizer (or J&J).
Higher antibody levels and more durable protection
jamanetwork.com/journals/jama/…
cdc.gov/mmwr/volumes/7…
Higher antibody levels and more durable protection
jamanetwork.com/journals/jama/…
cdc.gov/mmwr/volumes/7…
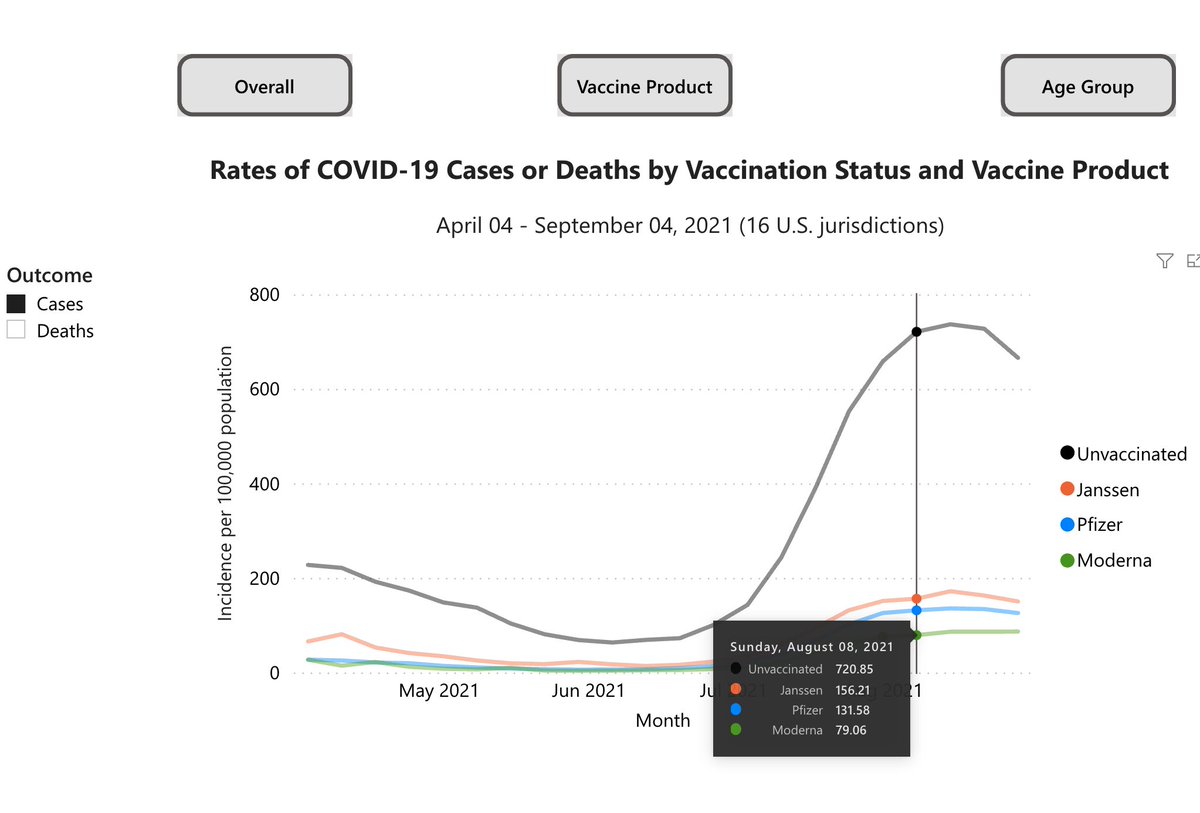
2/ Waning vaccine immunity is still debated in US populations, particularly unclear for Moderna.
See medrxiv.org/content/10.110…
comments here:
See medrxiv.org/content/10.110…
comments here:
https://twitter.com/profshanecrotty/status/1450183182175719424?s=21
There is certainly a range of studies indicating waning immunity, and I think there is some waning over time, particularly against Delta. But, I still also put a lot of weight on the actual clinical trial data: 
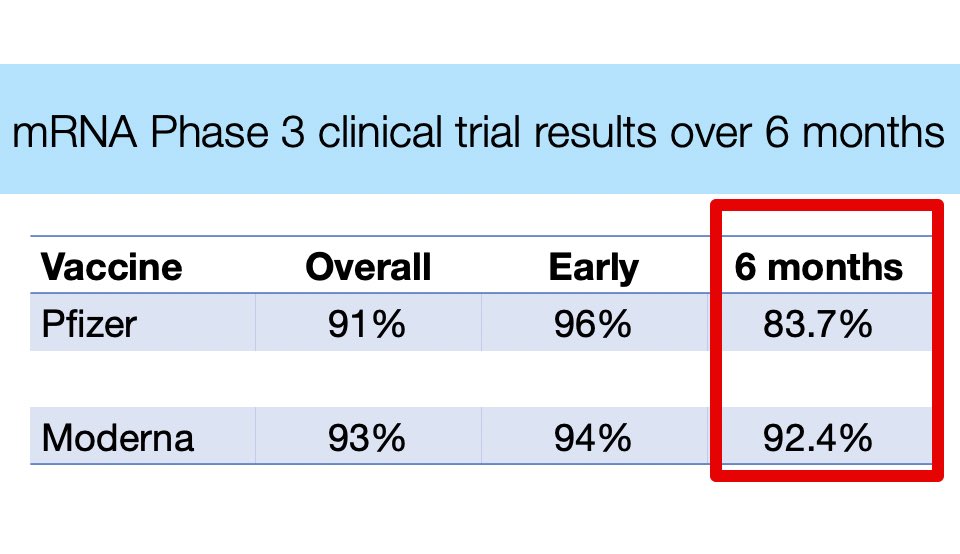
With all of that in mind, I think recommending boosters for high risk groups immunized with Moderna is reasonable, but with somewhat less emphasis than for Pfizer.
Please be aware that this is not the majority opinion.
Please be aware that this is not the majority opinion.
Additional points to comment on:
What shortcomings were there in the Moderna booster FDA data?
Still sad to not see any T cell data for boosters. (For Moderna or Pfizer)
Still sad to not see any T cell data for boosters. (For Moderna or Pfizer)
There's not a lot of Moderna booster safety data (~170 people). The safety decision largely depends on a combination of 1) Moderna Phase 3 clinical trial, 2) lower Moderna booster dose, 3) Pfizer booster FDA filing, 4) Pfizer booster vaccine safety data in million of Israelis.
What about for people with hybrid immunity? A booster isn't needed.
(Important note: it only counts as hybrid immunity if you had a *CONFIRMED* COVID infection, by PCR or antibody test).
(Important note: it only counts as hybrid immunity if you had a *CONFIRMED* COVID infection, by PCR or antibody test).
As Paul Offit said:
If you have hybrid immunity, "I would call yourself a victor. Call it a victory and bow out."
If you have hybrid immunity, "I would call yourself a victor. Call it a victory and bow out."
• • •
Missing some Tweet in this thread? You can try to
force a refresh