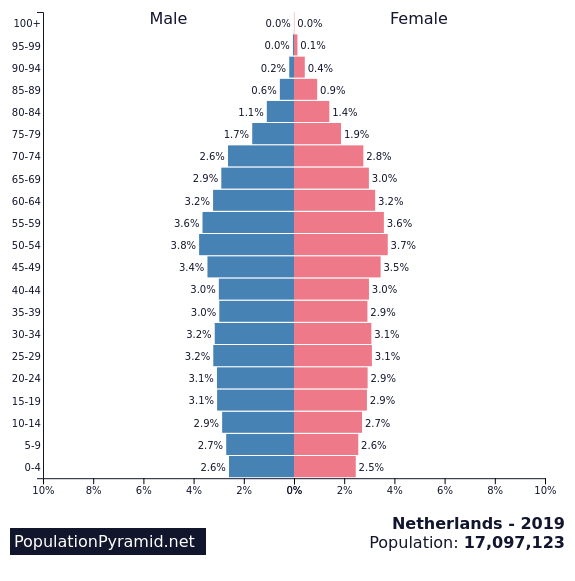
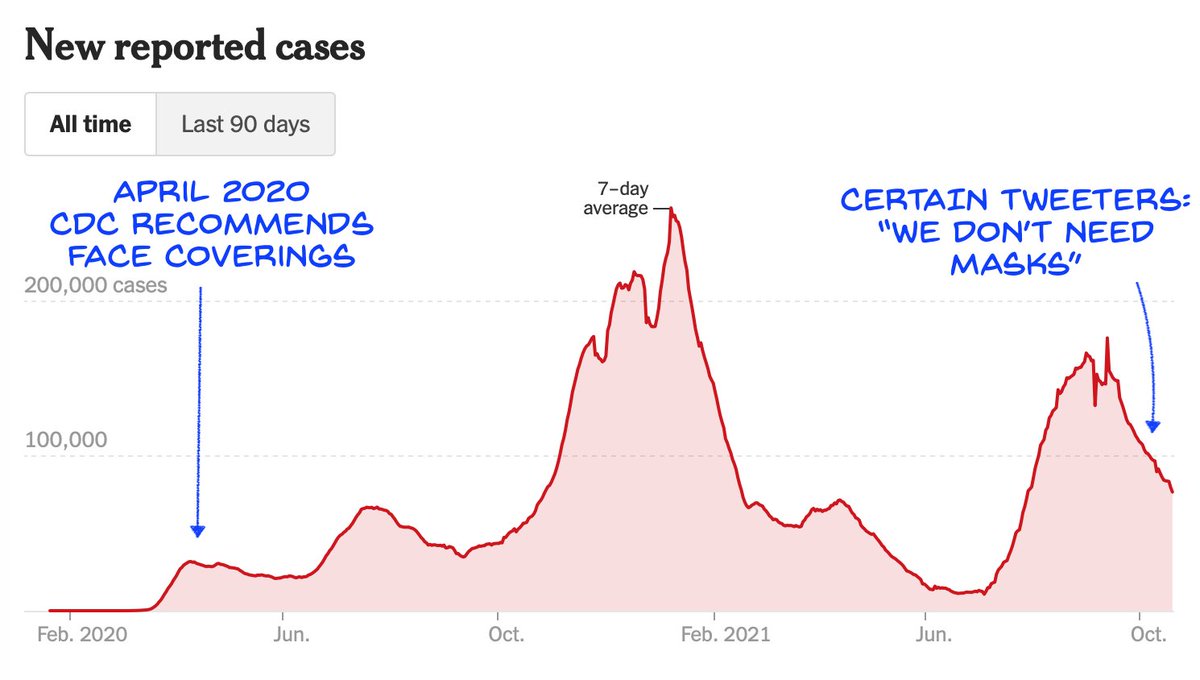
Pfizer's application for the vaccine for 5-11yo is progressing well.
FDA analysis identifies no red flags.
cnbc.com/2021/10/23/fda…
FDA analysis identifies no red flags.
cnbc.com/2021/10/23/fda…
VRBPAC meeting Tuesday to discuss risk/benefit ratio. Much will depend on the value of case suppression which I think should be considered in context of schools and families. Meeting materials at link below
fda.gov/advisory-commi…
fda.gov/advisory-commi…
Trial was too small (n ~ 1500 in vaccine group) to see myocarditis. FDA estimated benefits vs risks assuming MC rate similar to 12-17yo getting 30ug (maybe overestimate; 5-11yo get 10ug).
I think scenario 4 is most realistic. Scenario 5 uses CDC's observed CFR, but cases likely undercounted. Scenario 4 uses a wider observation network. Scenario 4 also uses the actual VE seen in the trial. Most cases in the trial were July-August, so were Delta. 

I'm most impressed that Pfizer seemed to have picked the Goldilocks dosage. 10ug was enough to get 90% VE against Delta, yet is only 1/3 of the dosage for ≥12yo
For the curious, the trips to the ICU for myocarditis probably involve intravenous immunoglobulin and steroids as rapid-acting anti-inflammatories
pediatrics.aappublications.org/content/pediat…
pediatrics.aappublications.org/content/pediat…
• • •
Missing some Tweet in this thread? You can try to
force a refresh