‘Who (what) is EU Notified Body Med19/#avct cld be working with’ to obtain EU IVD self use CE certificate. What EU state cld they be from, is it coincidental they are located in Spain, same city hospital area avacta used to verify AffiDX lft, Med19 have a Spainish website
1/15



1/15




On EU NANDO database from 18 Notified Bodies (NB) Med19/#avct could be working with ‘AEMPS Notified Body’ who certify med product devices under EU directive 98/79/EC for self use in-vitro diagnostic test (IVD). If not, then 17 other EU Notified Bodies to work with
2/15


2/15



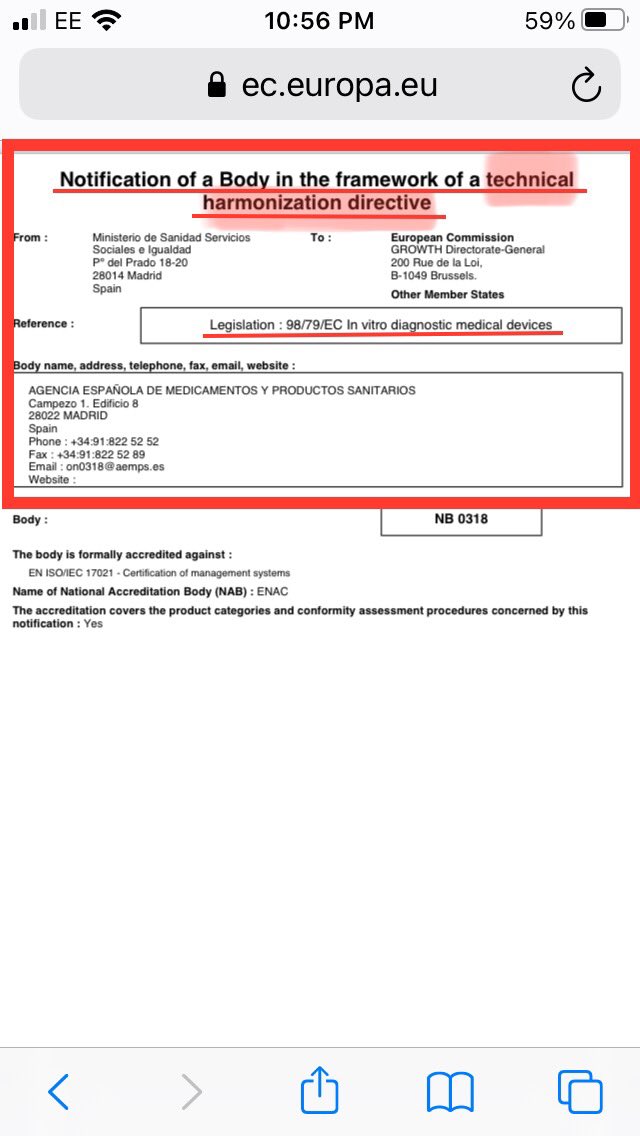
EU Notified Body much like mhra/fda. AEMPS NB is located in Spain/Madrid/Delgado. Same country, city, hospital area #avct used to independently verify AffiDX. AEMPS CE certified AffiDX for professional use, was application for AffiDX CE self use submitted around same time.
3/16



3/16




Both EU Notified Body & hospital used to independently verify AffiDX are max 5/6 miles from one another. Google map shows location distance. Think this isn’t a coincidence, has Med19 planned to validate lft in Spain. Cld be why AS said Med19 doing superb job in Q&A
#avct 4/15

#avct 4/15


All 18 Notified Bodies are accredited by EU, robustly checked for compliance and adhere to EU special directive 98/79/EC for IVD self use, and certify them. Notified Body/manufax requirements & scope of works in pics
#avct 5/15



#avct 5/15




Cont from post 5/15: Notified Body/manufax requirements & scope of works in pics, Is not exhaustive to list but can be much wider. Tweet 5-6 pics from EU commission doc 2021. Question & answers on how to obtain CE mark and certificate for #covid19 IVD LFT for self use
#avct 6/15

#avct 6/15


EU Notified body: Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) from Spain Madrid is one of 18 listed EU accredited Notified Bodies, who are on EU commissions NB Nando site. PDF shows scope to CE certify IVD for self use under EU 98/79/EC Directive
#avct 7/15



#avct 7/15




Wld be logical/easier for Med19 to use EU Notified Body in Spain Madrid. This only applies if Med19 submitted application before or on 25/05/21, after this date new 2017/746 regs apply. Med19 not applied by 25/05/21 they wld take IVDR 2017/746 route to CE mark AffiDX
#avct 8/15


#avct 8/15


Even if EU notified body AEMPS isn’t working with Med19 & #avct there is 17 others that can do same work. Med19 can only use one from EU 18 list. Spain hospital, Spain notified body, Med19 Spain website would be the perfect plan to obtain CE IVD self use certificate in EU
9/15



9/15




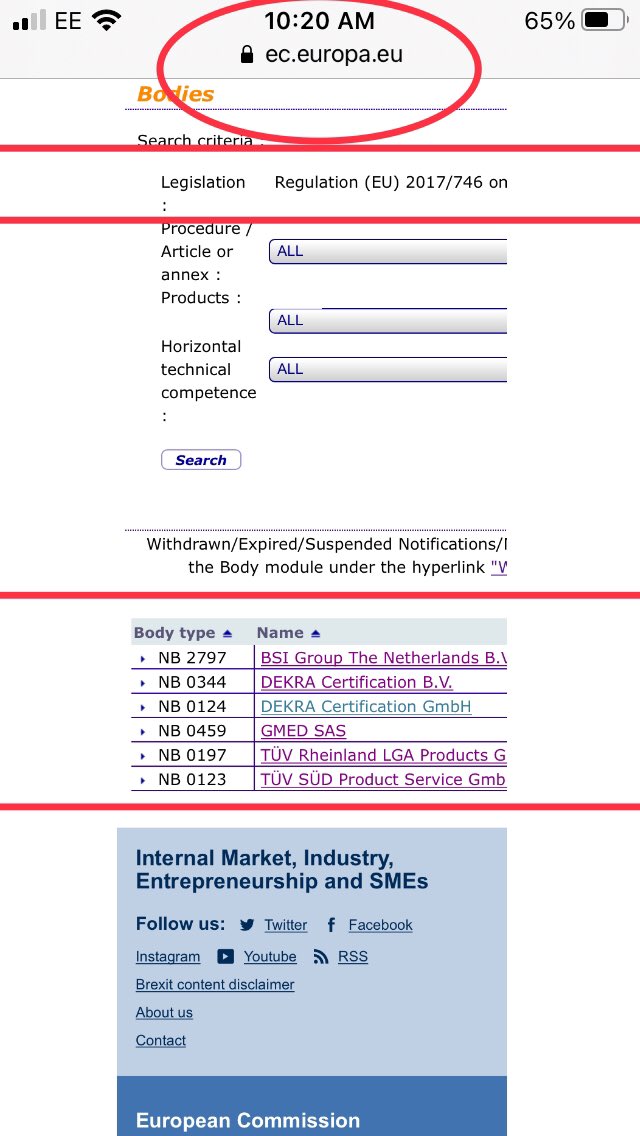
Tweets 1-9 apply if application for EU CE IVDD self use was before or on 25/05/21. If after 25 May then new regs IVDR EU 2017/746 apply. EU NANDO data base show only 6 Notified Bodies on list who certify under new regs IVDR EU 2017/746, Med19/#avct are using one of them
10/15

10/15

Med19 conducted submitted lay user study forming part of application to Notified Body on ease of use by non-professional (lay user) Going by medrxiv report which #avct did with Spain hospice users were non-professional. Med19 wld hav same trial success for self use CE mark
11/15

11/15


Almost certain UK MHRA will follow IVDR regs 217/746. Keeps UK aligned with EU, basically makes MHRA job easier. The fact regs 217/746 is robust in assessment of IVD tests, means EU Notified Bodies will have done all the hard work. EU/CE mark carries weight globally
#avct 12/15
#avct 12/15

IVDR EU regulation 217/746 would be the best route. Receiving CE mark for self use under this default regulation will in-force 217/746 into all 27 EU states, regardless of individual states own policy & laws regarding in-vitro diagnostic (IVD) tests for self use
#avct 13/15
#avct 13/15
Which ever route taken AS is determined 2 get EU CE for self use. He shld be, why!? because it opens up global markets for #avct AffiDX “Lets not forget CE Mark has been historically the passport for approval in majority of international markets” Gold pass 2 global markets 14/15 







And looks like EU CE mark certificate for home use is finally granted. No more waiting, second guessing, what ifs any more Med19 #avct find themselves in a unique position to take lion share of self use market. Certain they will do and smash it
@downunderfutbol👇👌👍🙂15/15



@downunderfutbol👇👌👍🙂15/15




• • •
Missing some Tweet in this thread? You can try to
force a refresh





