
Here's how mutations in #SARSCoV2 Nu variant (B.1.1.529) will affect polyclonal and monoclonal antibodies targeting RBD. These assessments based on deep-mutational scanning experiments; underlying data can be explored interactively at jbloomlab.github.io/SARS2_RBD_Ab_e… (1/n)
First, Nu variant has lot of antigenic change. Below are how mutations relate to escape averaged over 36 human antibodies. Many mutations at peak escape sites, especially E484, G446, K417, & Q493. This means even in polyclonal mix, lot of RBD antibodies will be affected. (2/n) 

Another way to assess polyclonal escape is how many epitope classes affected (nature.com/articles/s4146…). We do this using epitope scheme of @bjorkmanlab @cobarnes27 as adopted by @AllieGreaney. In this scheme, three potently neutralizing epitopes: class 1, 2, class 3. (3/n)
Unfortunately, the Nu variant has major escape mutations in each of these three epitope classes, as shown below. (The class 4 epitope less affected, but such antibodies also less potently neutralizing.) (4/n) 

Importantly, this does *not* mean Nu variant will fully escape vaccine- or infection-elicited antibodies. @PaulBieniasz @theodora_nyc have shown takes many many mutations to fully escape neutralization (nature.com/articles/s4158…), & there are also T-cells, non-neut Abs, etc. (5/n)
But I'd expect the Nu variant to cause more of a hit on vaccine- and infection-elicited antibody neutralization than anything we've seen so far. (6/n)
We can also look at some key monoclonal antibodies. the REGEN-COV cocktail is likely to take a hit for the Nu variant, especially the REGN10987 component. (7/n) 
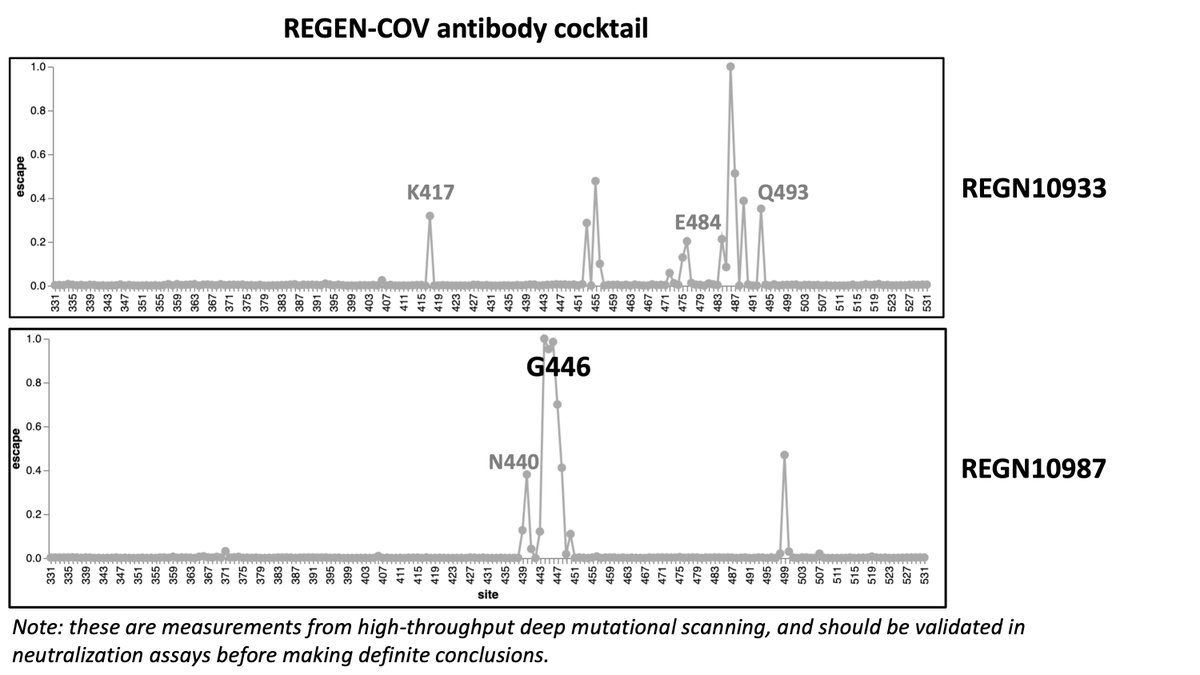
The early Eli Lily antibodies like LY-CoV555 (bamlanivimab) and LY-CoV016, which were already in trouble with current variants, aren't going to do any better against the Nu variant. (8/n) 

However, it appears the AstraZeneca AZD7442 cocktail and Vir's S309 are likely to hold up better against the Nu variant. See below. (9/n) 



You can explore other antibodies that might be of interest to you at jbloomlab.github.io/SARS2_RBD_Ab_e…. Importantly, all above results from high-throughput deep mutational scanning, and need to be validated in traditional experiments for high confidence. (10/n)
Also note large antigenic change does not mean Nu will necessarily spread & outcompete other variants. That will also depend on its transmissibility, which has been discussed by @Tuliodna & others (eg,
https://twitter.com/Tuliodna/status/1463911571176968194) and for which data remains preliminary. (11/n)
As @trvrb discussed in excellent recent thread, selection on variants so far may be dominated more by transmissibility than antigenic selection (
https://twitter.com/trvrb/status/1462816217794695170). But I'm not as sanguine that antigenic selection isn't also playing substantial role... (12/n)
Reason I say that is comparison of Nu variant to BANAL-20-52, a SARS-related CoV isolated from bats. If we compare both BANAL-20-52 and Nu to Wuhan-Hu-1, Nu has *many* more mutations that strongly affect antigenicity (
https://twitter.com/jbloom_lab/status/1440309655087902720). (13/n)
If selection was mostly for transmissibility, I'd expect sites of divergence of Nu and BANAL-20-52 relative to Wuhan-Hu-1 to perhaps be similarly distributed with respect to antigenic sites. But instead, Nu mutations much more focused in major antigenic sites. (14/n)
We can also use deep mutational scanning to assess how mutations in Nu affect ACE2 affinity (
https://twitter.com/jbloom_lab/status/1463879246875529224). But I suspect works less well than for antigenic mutations discussed above as there's lot more mutational epistasis for ACE2 affinity (eg, N501 & Q498). (15/n)
Important caveat: all of above is based on deep mutational scanning experiments. I'm sure more Nu-specific data will emerge in coming weeks to months. But I think it's useful to use prospective data we already have to calibrate what to expect. (16/n)
Thanks to @AllieGreaney @tylernstarr for doing deep mutational scanning on which above is predicated, & @NussenzweigL @VUMC_Vaccines @seth_zost @Vir_Biotech for sharing the antibodies. And of course the scientists providing rapid information about Nu (
https://twitter.com/Tuliodna/status/1463911554538160130).
And probably I should have used the variant name B.1.1.529 throughout above thread...
https://twitter.com/theosanderson/status/1464007031174619143
For people interested in G446 mutations, I'm going to link back to some details on this site. Here is an old thread discussing how G446 is a major site of escape in the class 3 epitope where (as of March 2021) mutations were not prevalent:
https://twitter.com/jbloom_lab/status/1372669322657550337
Also, here are old data showing how for a minority of convalescent individuals, the epitope centered on G446 is immunodominant with respect to serum neutralization:
https://twitter.com/jbloom_lab/status/1359136451875598345
Also, checkout the awesome CoV-RDB database of Bob Shafer, @Philip_Tzou, @sergeilkp, K Tao which compiles experimental data on G446 (and also whatever other mutation you care about): covdb.stanford.edu/search-drdb/?h…
Here are data from one of @AllieGreaney's papers (Fig 5 of science.org/doi/10.1126/sc…) showing that a K417-G446-E484 triple mutant sometimes but not always fully escapes neutralization by RBD-targeted antibodies, and effect is worse for convalescent than mRNA-1273 vaccine sera. 

Also adding this as another highly informed view just for balance on question of possible extent of antibody neutralization escape:
https://twitter.com/theodora_nyc/status/1464257411582148608
• • •
Missing some Tweet in this thread? You can try to
force a refresh





