
🔊Our latest @WHO living guidance on treatments for #COVID19, out today:
For severe/critical:
✅steroids
✅IL-6 RB
🟨mAb
🟧convalescent plasma🔥
For non-severe:
🟨mAb
🟧steroids
🚫convalescent plasma🔥
For both:
🟧remdesivir
🟧ivermectin
🚫HCQ
🚫LPV/r
bit.ly/3pAK6Oe

For severe/critical:
✅steroids
✅IL-6 RB
🟨mAb
🟧convalescent plasma🔥
For non-severe:
🟨mAb
🟧steroids
🚫convalescent plasma🔥
For both:
🟧remdesivir
🟧ivermectin
🚫HCQ
🚫LPV/r
bit.ly/3pAK6Oe


Two recommendations:
🌟against administering convalescent plasma for non-severe covid-19.
🌟against administering convalescent plasma for severe or critical covid-19, except in the context of a clinical trial.
🌟against administering convalescent plasma for non-severe covid-19.
🌟against administering convalescent plasma for severe or critical covid-19, except in the context of a clinical trial.

A living systematic review & network meta-analysis, led by @RSiemieniuk & @rominabrigpet with contributions from @jessbartoszko, @DenaZera, @AnilaQasim, @Elena_Kum et al, informed recommendations.
Now with a 🔥🔥 website with evidence readily accessible: covid19lnma.com.
Now with a 🔥🔥 website with evidence readily accessible: covid19lnma.com.

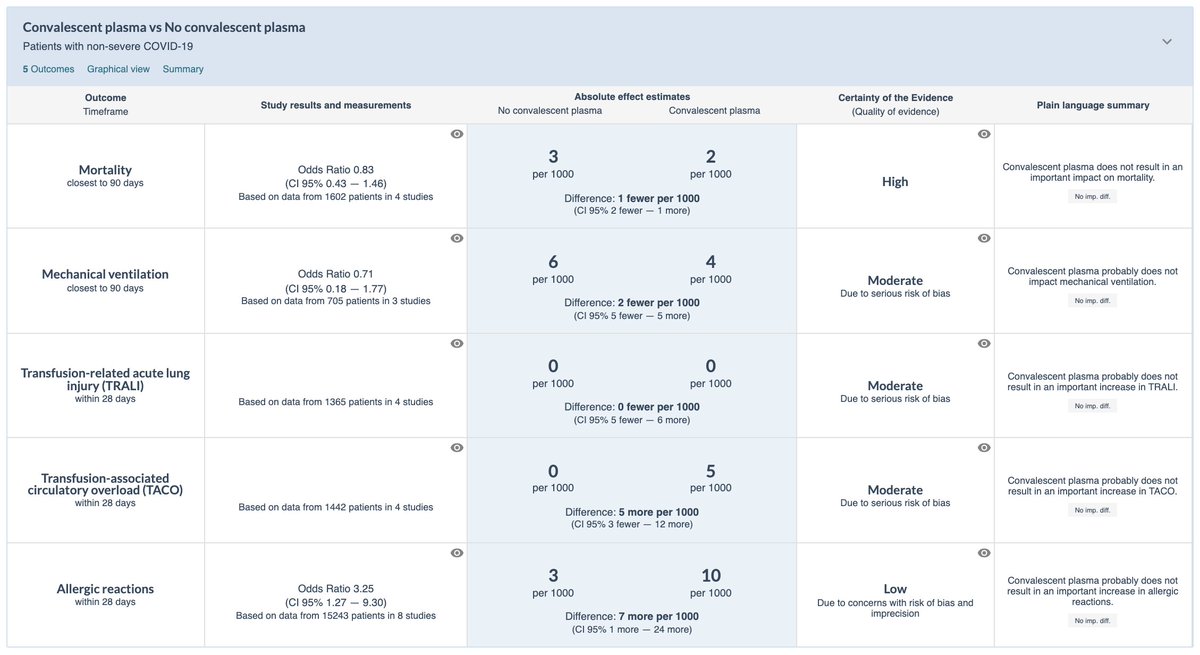
🌟For non-severe illness:
- No benefit for critical or important outcomes, including mortality and mechanical ventilation
- Potential for harm
- Resource requirements / practical issues (donor identification & testing, plasma collection / storage / transportation, administration)


- No benefit for critical or important outcomes, including mortality and mechanical ventilation
- Potential for harm
- Resource requirements / practical issues (donor identification & testing, plasma collection / storage / transportation, administration)


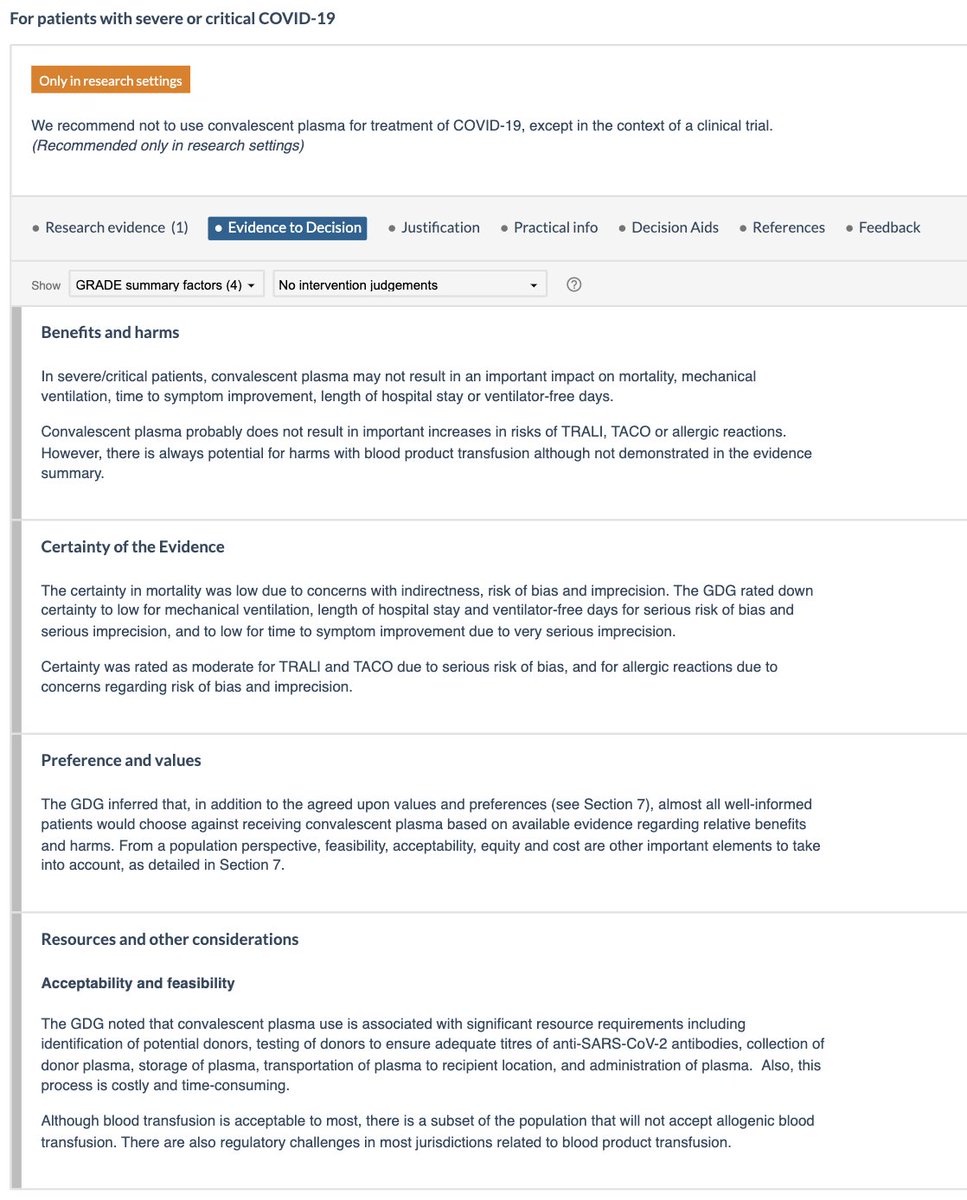
🌟For severe/critical illness:
- Low certainty evidence for mortality, mechanical ventilation, etc.
- Potential for harm.
- Resource requirements (similar issues, though may be easier to administer in hospitalized patients).
- More research needed to reduce uncertainty.


- Low certainty evidence for mortality, mechanical ventilation, etc.
- Potential for harm.
- Resource requirements (similar issues, though may be easier to administer in hospitalized patients).
- More research needed to reduce uncertainty.


This work contributes to collaborative living guidance produced by @WHO and the #MAGIC Evidence Ecosystem on drugs to prevent #COVID19, also published via @bmj_latest.
This is an incredible cross-collaborative effort - honoured and lucky to be part of it.
This is an incredible cross-collaborative effort - honoured and lucky to be part of it.
Associated guideline in the @WHO portal: bit.ly/3ExWeG3
Associated guideline in @theMAGICapp:
bit.ly/3Gk5ldZ
Associated LNMA: bit.ly/3lIKNnP
Associated guideline in @theMAGICapp:
bit.ly/3Gk5ldZ
Associated LNMA: bit.ly/3lIKNnP

Important to recognize the superstar team behind this work:
@Bram_Rochwerg
@RSiemieniuk
@ThomasAgoritsas
@LamontagneFran5
@LisaAskie
@lyubovlytvyn
@drhelmac
@drwagdy
@erlinaburhan
@DrMCecconi
@HeikeGeduld
@PRICE_critcare
@bhwords
@leticiakawano
@arthurkwizera
@Gremile
@Bram_Rochwerg
@RSiemieniuk
@ThomasAgoritsas
@LamontagneFran5
@LisaAskie
@lyubovlytvyn
@drhelmac
@drwagdy
@erlinaburhan
@DrMCecconi
@HeikeGeduld
@PRICE_critcare
@bhwords
@leticiakawano
@arthurkwizera
@Gremile
(continued)
@Nsutebu
@NidaQadirMD
@saniyasbz
@msh_manu
@sebasugarte
@sridhartweet
@VuyisekaDubula
@ananda1958
@DenaZera
@jessbartoszko
@rominabrigpet
@GuyattGH
@diazjv
@PerVandvik
@Nsutebu
@NidaQadirMD
@saniyasbz
@msh_manu
@sebasugarte
@sridhartweet
@VuyisekaDubula
@ananda1958
@DenaZera
@jessbartoszko
@rominabrigpet
@GuyattGH
@diazjv
@PerVandvik
• • •
Missing some Tweet in this thread? You can try to
force a refresh



