So excited to be a part of this important study led by @michelle_monje on how significant longterm neurologic damage can occur after a mild respiratory-only SARS-CoV-2 infection. My own🧵on the findings of this study with relevance to #longCovid (1/)
biorxiv.org/content/10.110…
biorxiv.org/content/10.110…
How can a mild respiratory SARS-CoV-2 infection lead to longterm neurological symptoms? Possibilities include 1) direct infection of 🧠, 2) autoimmunity, and 3) inflammatory impact of infection distal to the 🧠. In this study, we focused on 3) 👇🏽 (2/) 
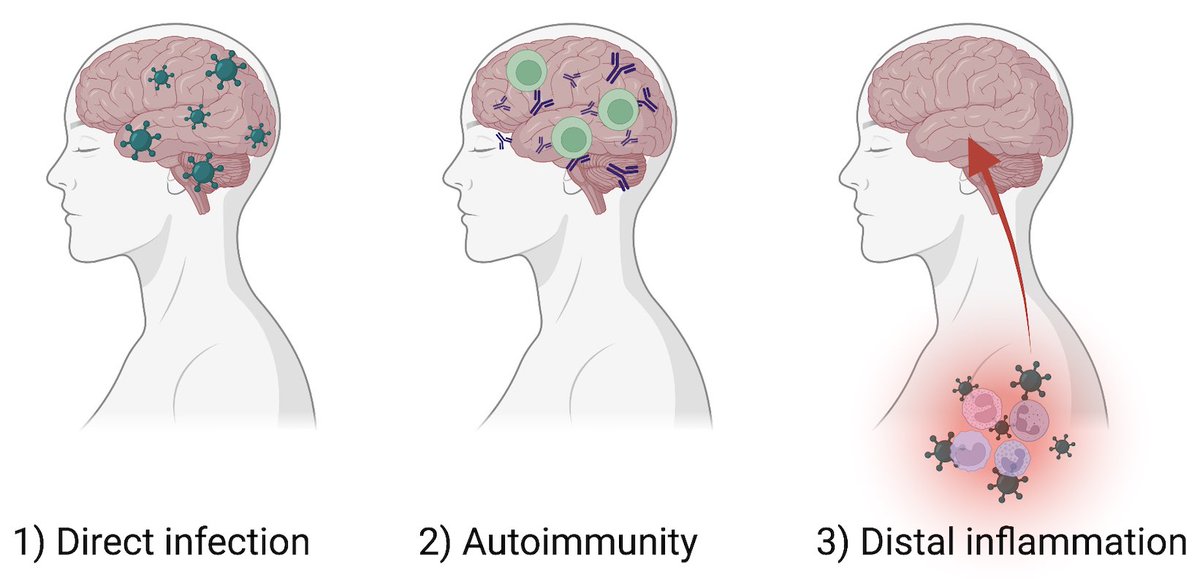
To achieve this goal, @peowenlu & @ericsongg used a mouse model developed by @BenIsraelow & @ericsongg in which we can control where the infection happens. Using AAV-hACE2 intratracheally, we can confine the SARS-CoV-2 infection only to the lungs. (3/)
rupress.org/jem/article/21…
rupress.org/jem/article/21…
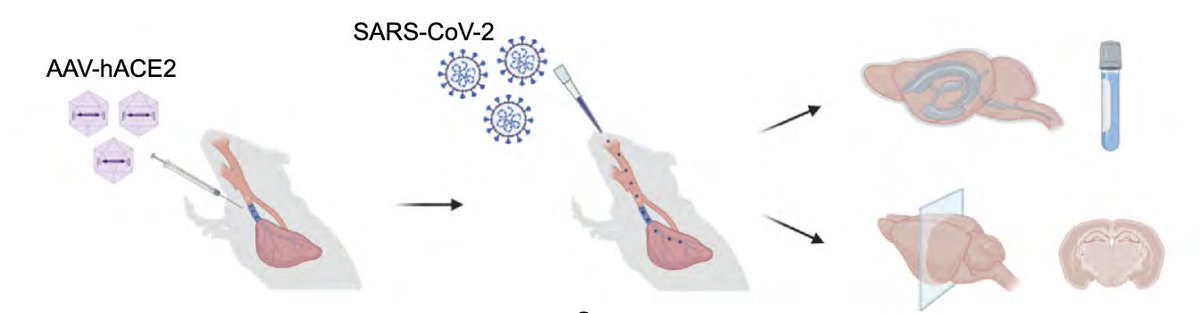
In fact, mice infected only in the respiratory tract show no evidence for weight loss (a disease measurement)(B),and no evidence of SARS-CoV-2 infection in the brain (C). (4/) 
So what do systemic (serum) and local (cerebrospinal fluid; CSF) cytokines look like after this mild respiratory-only infection? Not surprisingly, we see many elevated cytokines 7 days after infection in the serum. We also see elevated cytokines in the CSF. (5/) 
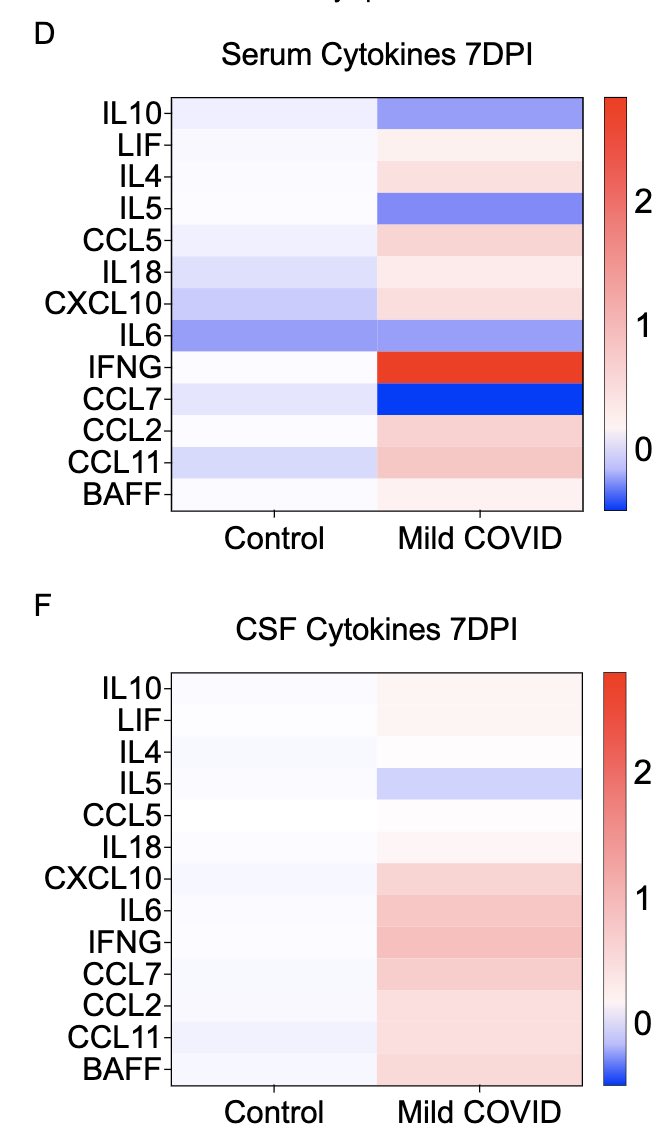
Somewhat unexpectedly, we also see that some of these cytokines remain elevated at 7 weeks post infection in both sera and CSF. Similarly elevated cytokines have been reported from the sera of long COVID patients by others, months after primary infection. (6/) 
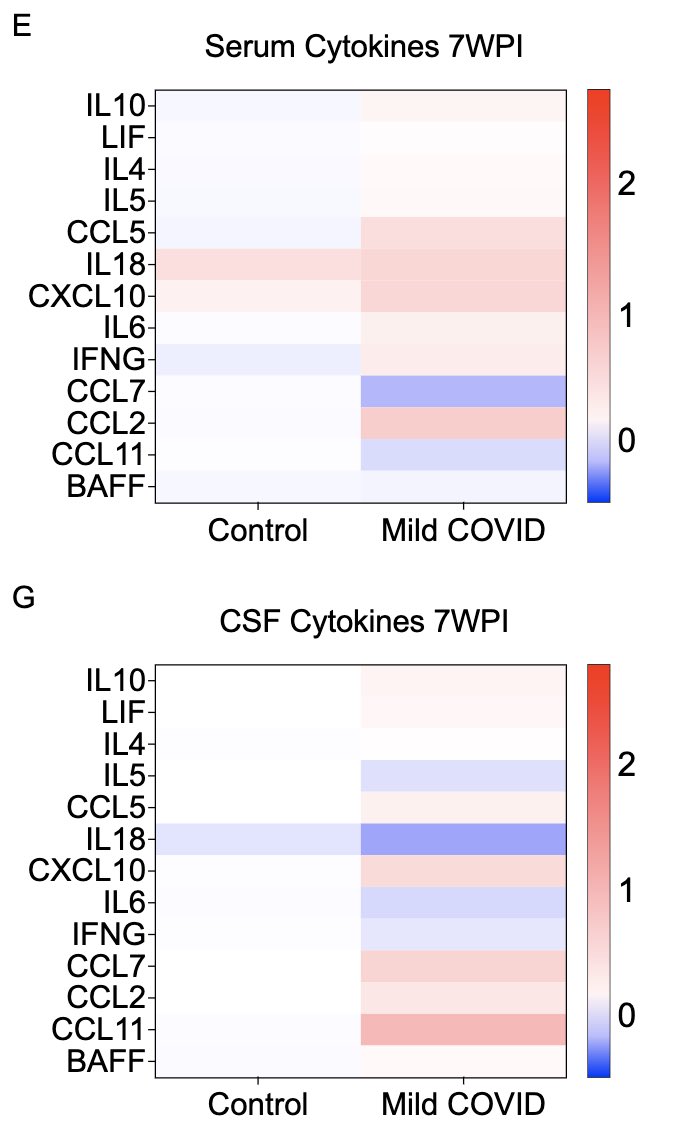
What does a respiratory-only mild COVID do to the brain? @ThisIsAnthonyFC and @AnnaGeraghty2 examined the subcortical white matter of two independent strains of mice and found consistently increased microglial reactivity at 7 days and 7 weeks post infection. (7/) 
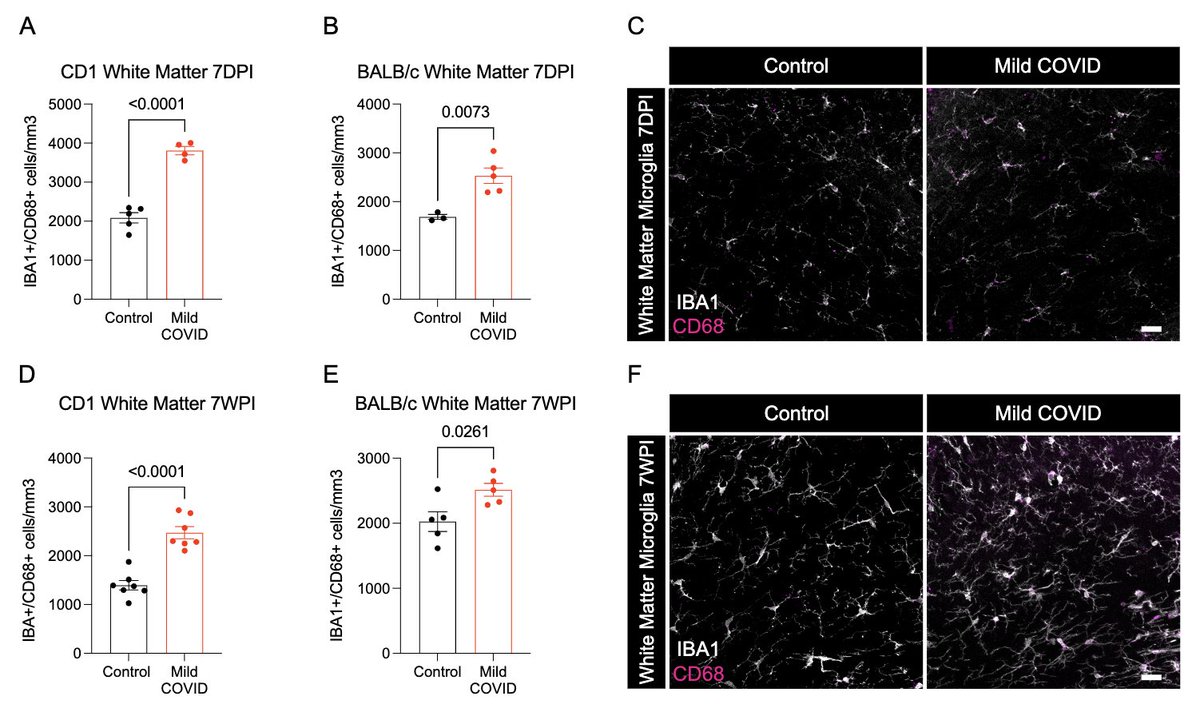
Next, with @nathavindra, autopsies from 9 individuals found to be SARS-CoV-2-positive by nasal swab PCR at the time of death were examined. Brains from those with even mild or asymptomatic SARS-CoV-2 infection had microglial reactivity in subcortical white matter. (8/) 
Neurogenesis in the hippocampus is thought to support memory function. Reactive microglia can impair this process. Indeed, mice that had mild SARS-CoV-2 infection 7 days or 7 weeks prior had significantly lower # of neuroblasts than controls. This could ⬇️ memory function. (9/) 
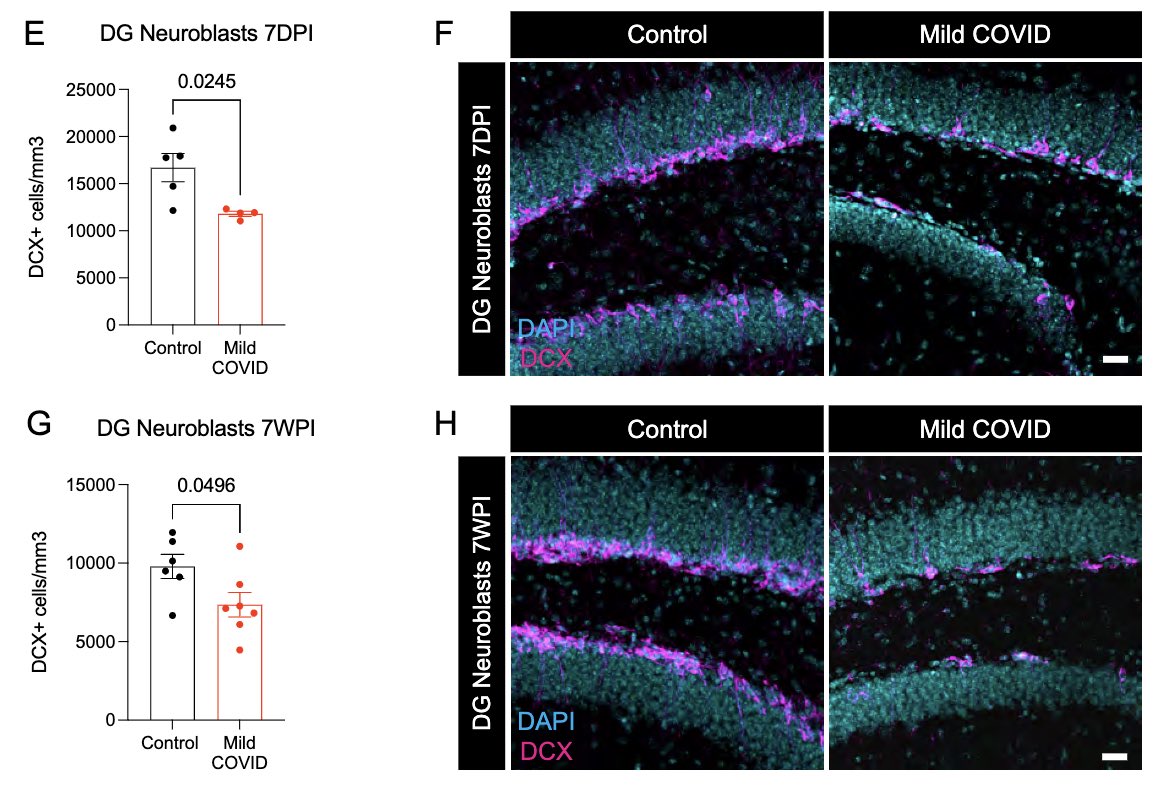
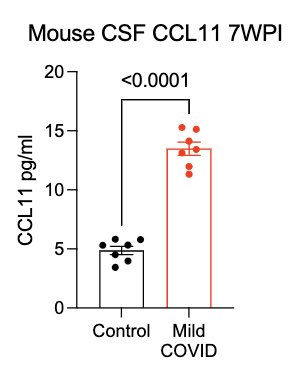
What can lead to impaired neurogenesis in hippocampus? We looked into a chemokine called CCL11 (eotaxin-1) which was shown to reduce neurogenesis (Villeda et al). In our mice, CCL11 was elevated in the CSF 7 weeks after mild respiratory infection. (10/)
pubmed.ncbi.nlm.nih.gov/21886162/
pubmed.ncbi.nlm.nih.gov/21886162/
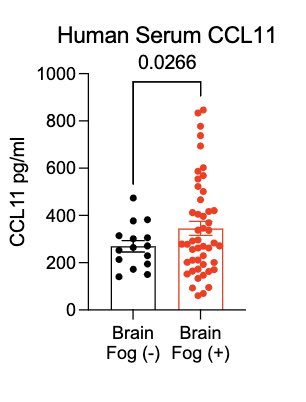
In collaboration with @PutrinoLab at @MountSinaiNYC, we found significantly elevated circulating levels of CCL11 in long COVID patients who reported brain fog vs. those who did not. Many 🙏🏼 to @wood_jamie_1 @LauraTabacof @GeneticHeartDoc #DaynaMcCarthy & the patients! (11/) 
What other changes are happening in the brain of mice with mild respiratory infection? Within just 7 days of infection, we found a loss of ~1/3 of oligodendrocytes, which persisted for at least 7 weeks! Analysis by @ThisIsAnthonyFC and @AnnaGeraghty2 (12/) 
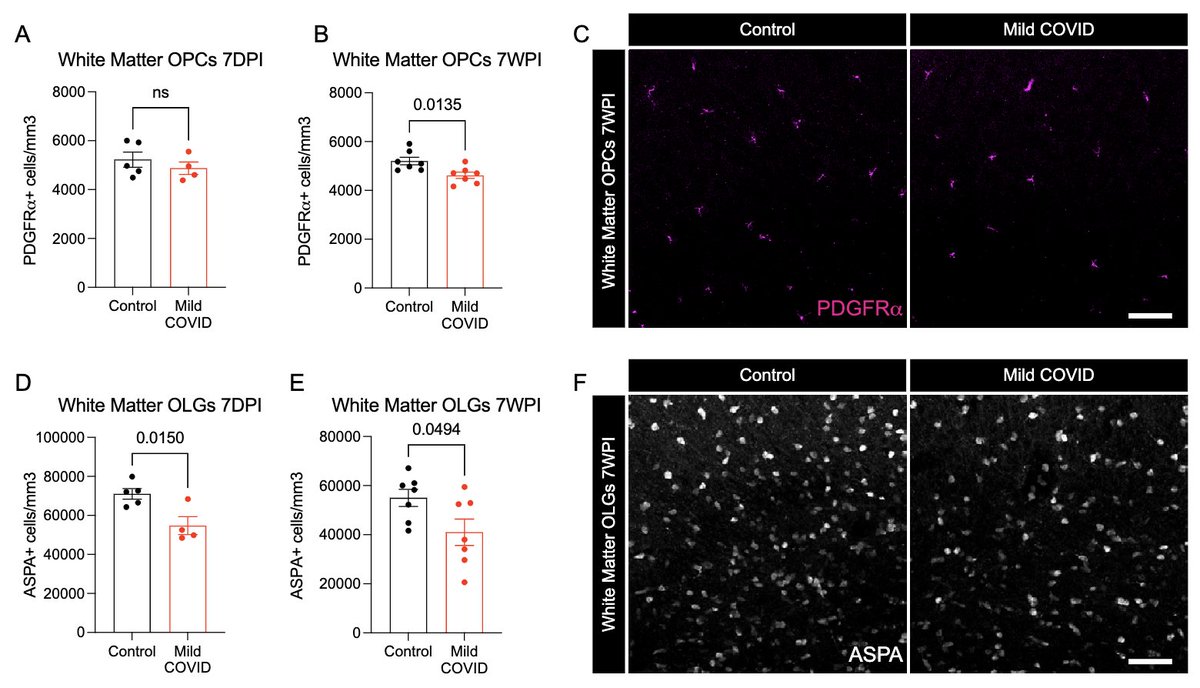
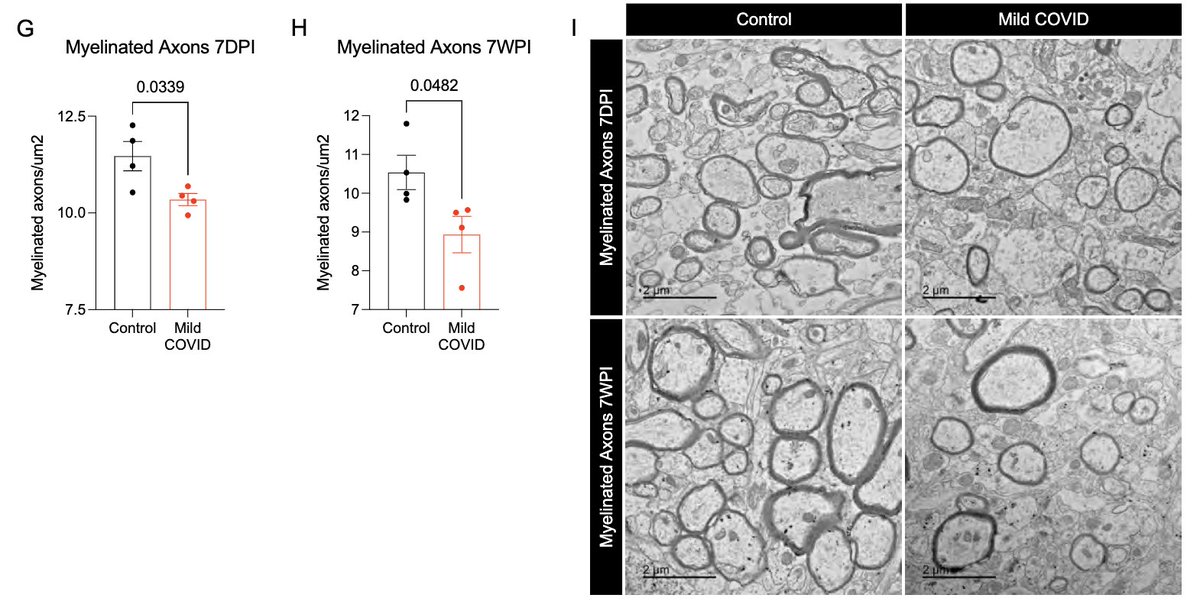
This loss of oligodendrocytes was accompanied by reduced myelinated axon density in subcortical white matter within 7 days of infection. This could lead to ⬇️ neural circuit function, axon health and to numerous deleterious neurological consequences of SARS-CoV-2 infection. (13/) 
This study was led and executed by my amazing colleague, @michelle_monje & her team + @PutrinoLab @MountSinaiNYC, @nathavindra et al. From our Yale team, I wish to highlight @peowenlu @ericsongg and others listed here 💪🏼 (14/) 

In a nutshell, this study illustrates that respiratory-only mild SARS-CoV-2 infection can lead to detrimental changes in the brain, likely mediated by inflammatory factors. Similar neuropathobiology may be shared in chemo-brain, post-ICU syndrome and ME/CFS. (15/)
This study also opens up all kinds of questions and possibilities. For example, therapies that can 1) block inflammatory cytokines, 2) block inducers of such cytokines, or 3) reset reactive microglia can be considered for future clinical trials. Thank you for reading till end.
I highly recommend this very informative thread posted by @michelle_monje on this study.
https://twitter.com/michelle_monje/status/1480939466294370307
• • •
Missing some Tweet in this thread? You can try to
force a refresh