
New Lancaster lab publication out in @Nature! This was all @Dabrica’s idea and due to her ingenuity and hard work, with help from @ChiaradiaIlaria, @laupellegrini, and Alex Kalinka. Check it out and see our🧵below!
rdcu.be/cFi7f
rdcu.be/cFi7f
Male and female brains differ in their total brain volume. They also show differential susceptibility for some neuropsychiatric disorders. We sought to explore the developmental origin of these differences by generating brain #organoids from male and female stem cell lines. 

Adding to the cellular complement, we exposed organoids to sex steroids. Addition of androgens (testosterone and DHT) increased the numbers of basal progenitors and their proliferation while estrogen didn’t elicit an observable phenotype. 


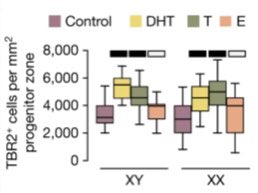
Viral labelling of radial glia showed that androgens promote symmetric divisions, thus increasing their pool. When DHT was removed from the media, the clone size returned to control levels. 

RNA Scope showed presence of androgen receptor in radial glia. Electroporations of constitutively active androgen receptor confirmed increased proliferation capacity of cells. Activating estrogen receptor did not mimic that process. 



Bulk and single cell RNA-seq showed involvement of HDACs and mTOR in the generation of androgen-induced phenotype. Drug interference and DHT-mediated rescue confirmed their involvement in androgen signalling. (Both pathways are thought to be involved in ASD) 



This happened in the dorsal brain organoids, where excitatory neurons are born. What about ventral, the source of interneurons? Ventral progenitors reacted differently to DHT, without observable increase in intermediate progenitors. Could this contribute to E/I imbalance? 

Pulse-chase experiments showed that androgen-increased progenitors resulted in an increase in excitatory neurons. This increase was comparable to the size differences observed between male and female total brain volumes. 

Androgens are present in the embryonic brain. At higher levels (in males) our data suggest they specifically increase certain progenitors leading to increased excitatory neurons. This could be one mechanism for the divergence in total brain volumes between males and females. 

• • •
Missing some Tweet in this thread? You can try to
force a refresh






