
Happy present our manuscript on targeting RTK and adaptive resistance in RTK-addicted sarcomas 🎯. @MLadanyi, Romel Somwar and amazing @sloan_kettering pediatrics team! @JGladeBender @MichaelVOrtiz #MSKkids #MolPath #Sarcoma @OncoAlert
cancerres.aacrjournals.org/content/early/…
🧵 🧵 🧵 (1/19)
cancerres.aacrjournals.org/content/early/…
🧵 🧵 🧵 (1/19)

Disclaimer #1: I have no conflicts of interest to disclose.
Disclaimer #2: Please note that this post does not constitute medical advice. Please consult your physician or other qualified health care provider. (2/19)
Disclaimer #2: Please note that this post does not constitute medical advice. Please consult your physician or other qualified health care provider. (2/19)
With the development and broader adaptation of NGS, we now know that a variety of soft tissue sarcomas harbor receptor tyrosine kinase (RTK) fusions. (3/19) 

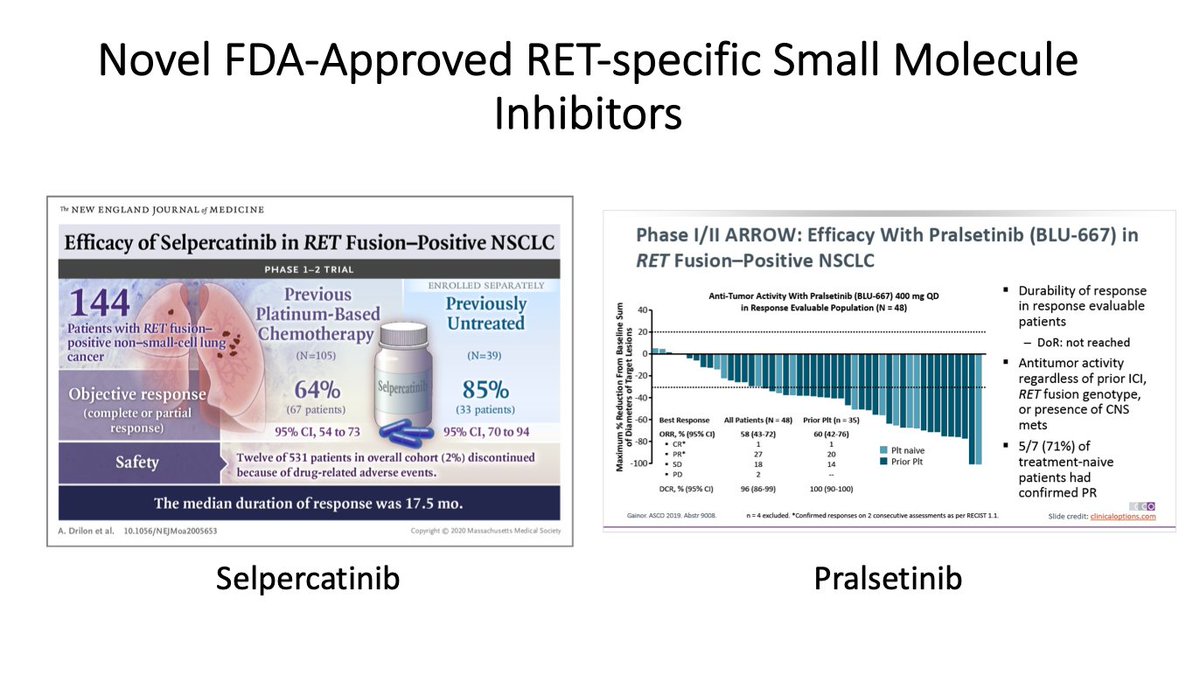
This finding is important, because we have specific RTK inhibitors that were shown to be effective in several RTK-driven tumors. For example, RET specific inhibitors are approved for thyroid and/or lung cancers with RET alterations. (4/19) 
Although there is early evidence for the clinical efficacy of RTK inhibitors in sarcoma (e.g. see the outstanding response in NTRK3-rearranged sarcoma in the attached image), the preclinical research is sparse due to the rarity of the disease and paucity of disease models. (5/19) 

Why do we need preclinical research, if we can just treat our patients? Unfortunately,most patients progress on RTKi therapy. In many cases we don’t know the cause of resistance. In the instances that we know,we can co-target the resistance pathway to prolong the response. (6/19)
Ultimately, our goals were to:
- Establish new RTK-dependent sarcoma models to foster research.
- Determine the efficacy of RTK inhibitors in the treatment of RTK-driven sarcomas.
- Elucidate mechanisms of drug resistance in order to devise better therapeutic strategies. (7/19)
- Establish new RTK-dependent sarcoma models to foster research.
- Determine the efficacy of RTK inhibitors in the treatment of RTK-driven sarcomas.
- Elucidate mechanisms of drug resistance in order to devise better therapeutic strategies. (7/19)
First,the models.We used an orthogonal approach: CRISPR-cas9 transformed human mesenchymal stem cells on one hand,and patient-derived cells and xenografts on the other.This way, we have tightly controlled conditions in the first case, and a real-world example in the second.(8/19)
We used human mesenchymal stem cells (HMSC) as the putative cell of origin of sarcomas, and modified them with CRISPR-cas9 to harbor a SPECC1L-RET fusion. SPECC1L-RET-positive HMSC cells (HMSC-RET) acquire an aggressive, atypical phenotype. (9/19) 

The patient-derived (PD) models (cells and xenograft) were established from a pediatric patient with a metastatic kidney sarcoma harboring an identical SPECC1L-RET fusion (SR-Sarc-0001). (10/19) 

Now, the therapy.We extensively characterized the therapeutic response to multiple RET inhibitors.We show that while HMSC-RET and PD cells respond to low nanomolar concentrations of multiple RETi, much higher doses are required for parental HMSC cells. Therapeutic window! (11/19) 

How about in vivo? Xenograft tumors shrank to immeasurable disease in response to selpercatinib! After we stopped treatment, the tumors took 42 days to regrow. In HMSC-RET xenografts, the therapy reduced tumor to small remnants, composed of acellular stroma – a scar. (12/19) 

Models ✅ Therapy ✅
Now to therapy resistance. To start, we concentrated on the signaling pathways mostly affected by the therapy. Phospho-proteomic and transcriptomic analysis showed a prominent role of the MAPK pathway in the therapeutic response. (13/19)
Now to therapy resistance. To start, we concentrated on the signaling pathways mostly affected by the therapy. Phospho-proteomic and transcriptomic analysis showed a prominent role of the MAPK pathway in the therapeutic response. (13/19)

To analyze MAPK pathway response, we looked at the temporal changes in the axis from top to bottom (RET-SHP2-MEK-ERK-P90RSK).Remarkably, we observed a striking reactivation of ERK, without a corresponding change in RET or SHP2.Something has changed downstream of RET-SHP2. (14/19) 

We hypothesized that negative regulators of ERK, the DUSP phosphatases, are downregulated, causing a rebound of phospho-ERK. Indeed, there is a consistent temporal correlation between ERK reactivation and downregulation of DUSPs. Is the cancer trying to circumvent RET inhibition? 

If this model is correct, can we enhance RET therapy by employing combined RET+ERK inhibition? We employed a clinical stage ERK inhibitor ulixertinib and combined it with RETi. In vitro, this combination is synergistic. In vivo, better tumor growth control was achieved. (16/19) 

Is this exclusive to RET fusions? We show here that sarcoma cells with another fusion, NTRK3 (yes, we established NTRK3 fusion sarcoma models too!), display a similar adaptation, expanding the applicability of our work. (17/19) 

To my non-sarcoma oncology colleagues: there is no reason to believe that these mechanisms are unique to soft tissue sarcomas. We hope that our work will prompt the research in the broader area of adaptive MAPK resistance. (18/19) #PrecisionMedicine
Kudos to @CR_AACR for an amazing editorial process. As always, we strive to support open access, so this work is freely available online.
Please consider donating to pediatric cancer research! Every small donation matters!
The end. (19/19)
Please consider donating to pediatric cancer research! Every small donation matters!
The end. (19/19)
• • •
Missing some Tweet in this thread? You can try to
force a refresh



