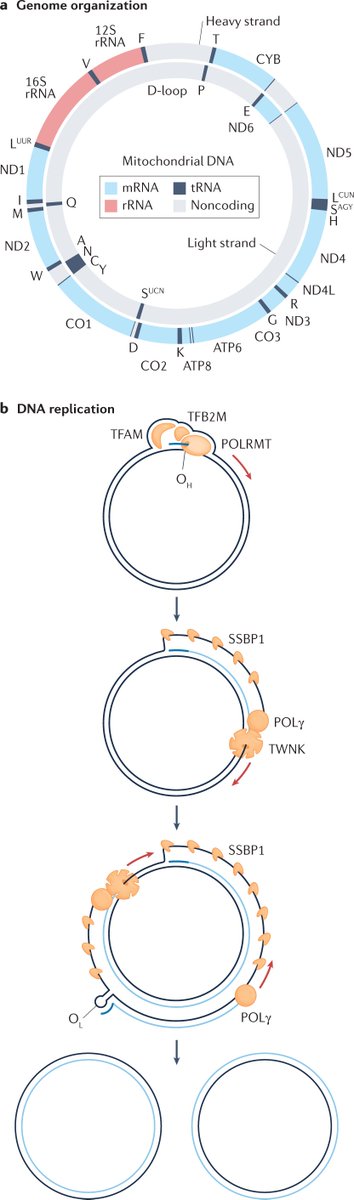
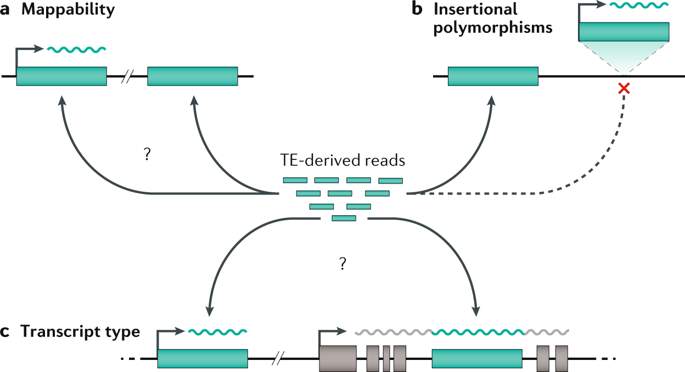
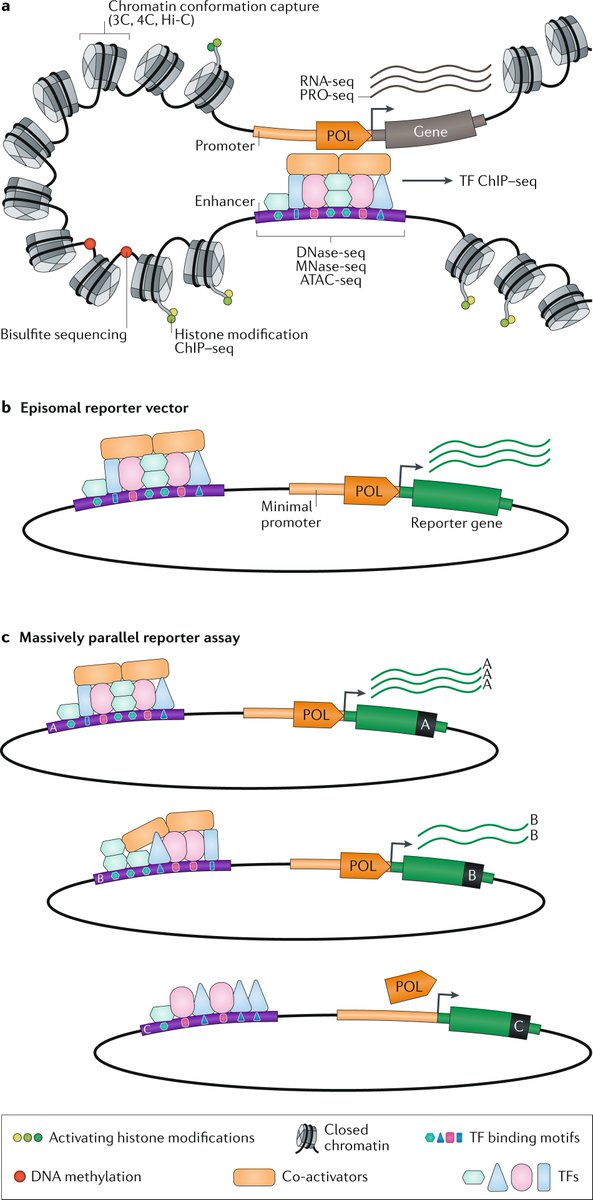
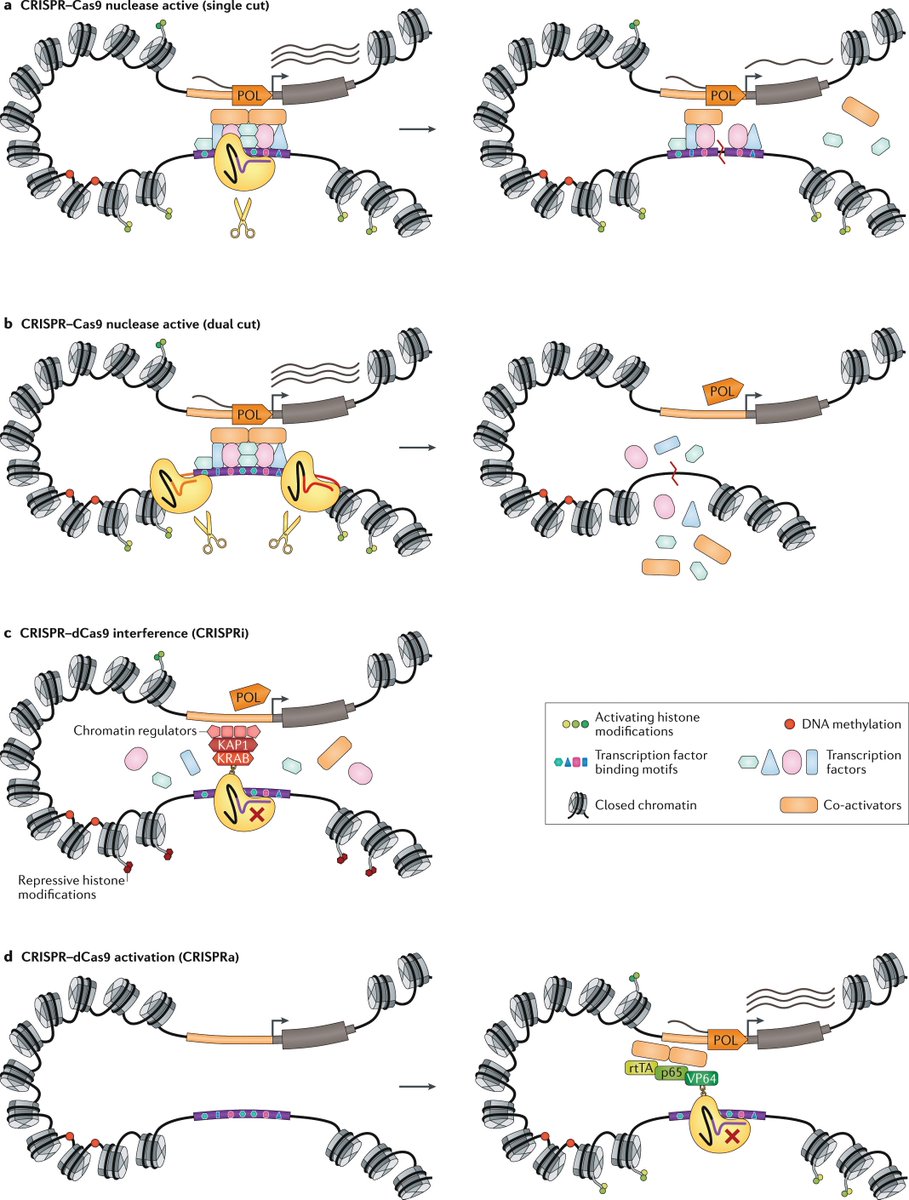
Lineage tracing meets single-cell omics: opportunities and challenges go.nature.com/2UPu2cl Our 2020 #Review by @danwagnerlab and @KleinLabHMS
@harvardmed @UCSF
@harvardmed @UCSF
A fundamental goal of developmental and stem cell biology is to map the developmental history (ontogeny) of differentiated cell types 

Recent advances in high-throughput single-cell sequencing technologies have enabled the construction of comprehensive transcriptional atlases of adult tissues and of developing embryos from measurements of up to millions of individual cells 

Parallel advances in sequencing-based lineage-tracing methods now facilitate the mapping of clonal relationships onto these landscapes and enable detailed comparisons between molecular and mitotic histories 
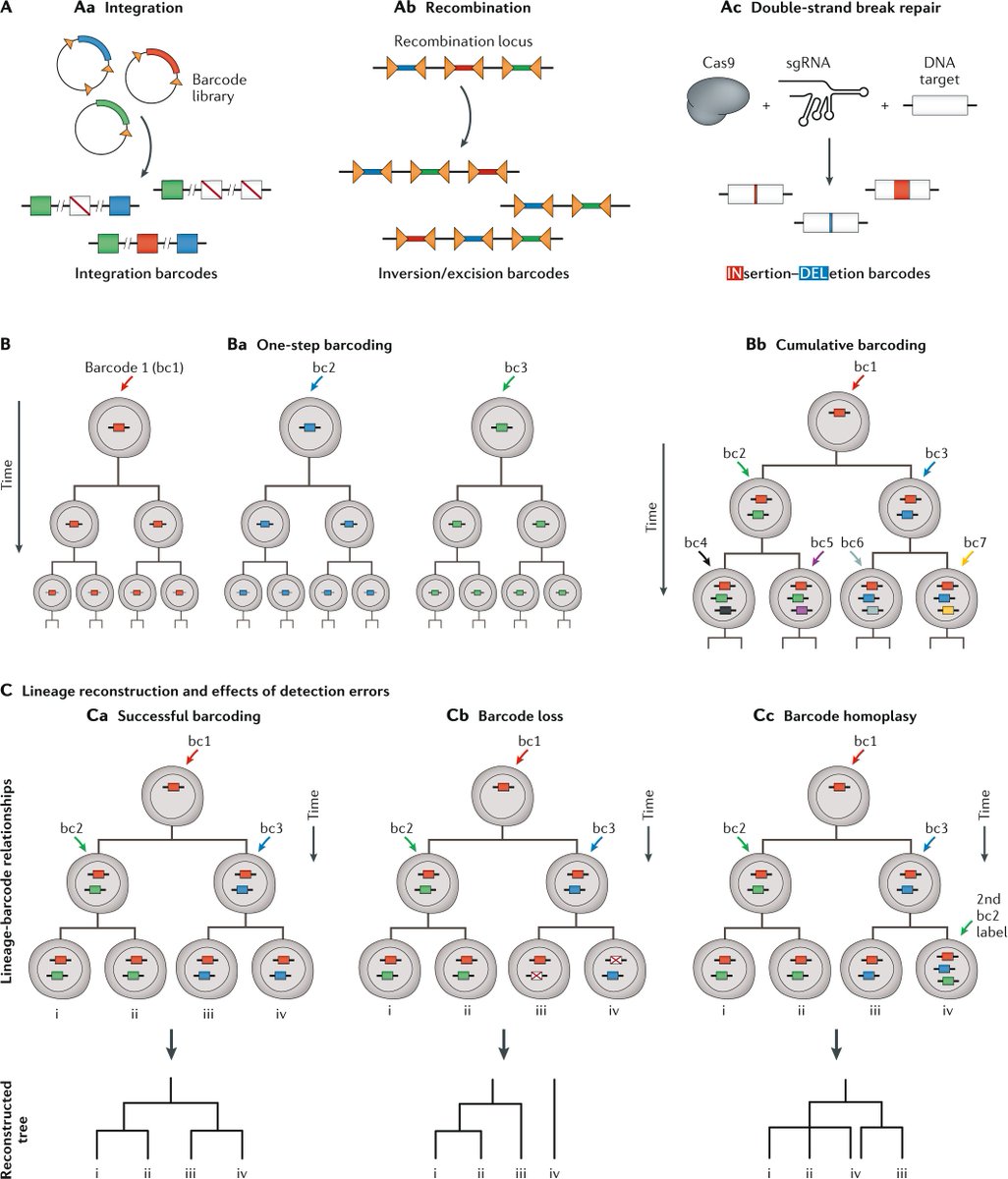
Here, the authors review recent progress and challenges, as well as the opportunities that emerge when these two complementary representations of cellular history are synthesized into integrated models of cell differentiation 
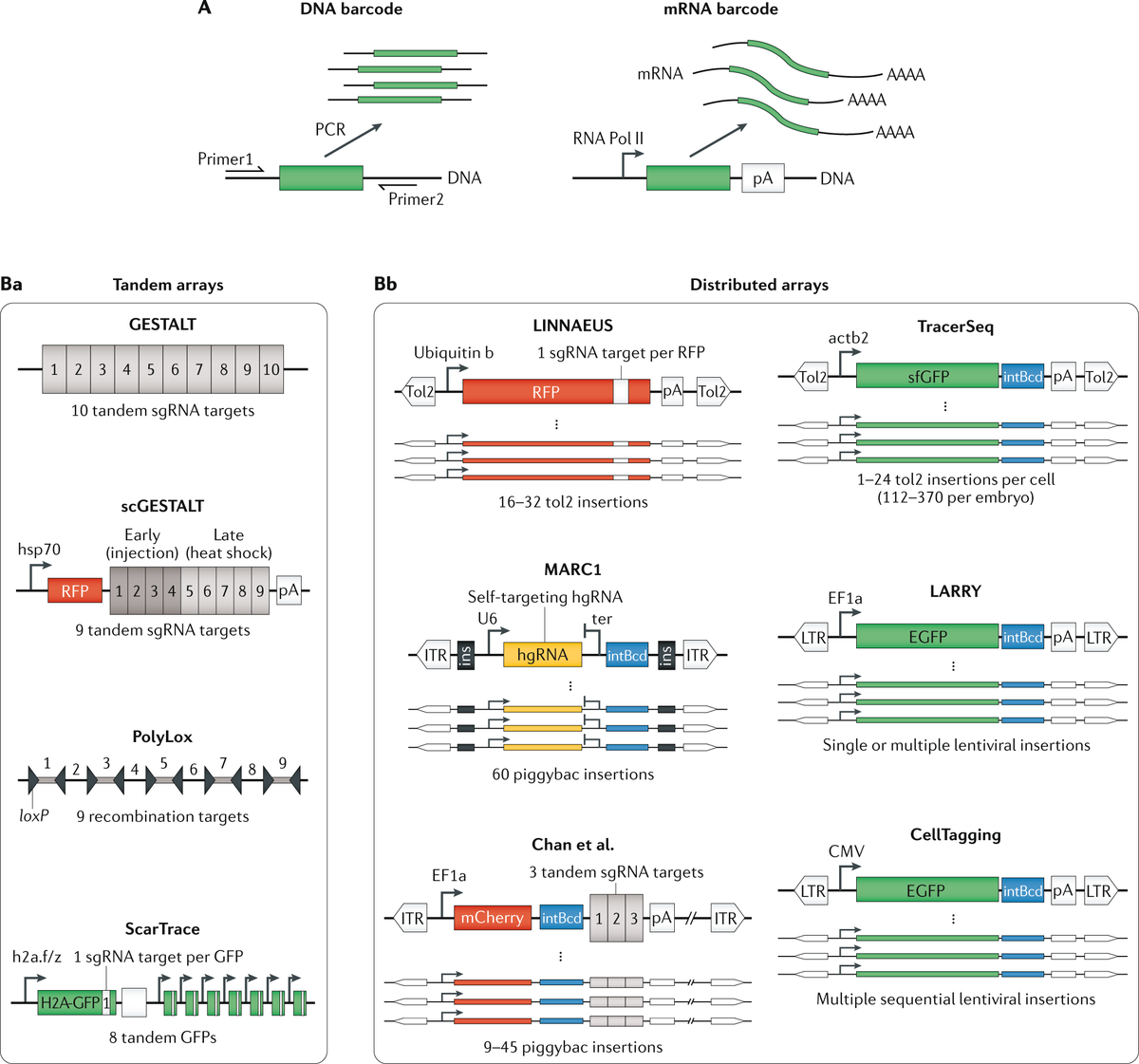
• • •
Missing some Tweet in this thread? You can try to
force a refresh