
🧵 Based on an analogy with AAm/Sty copolymers that exhibit #UCST properties we managed to replace Sty by aromatic ring-containing CKAs (MPDL & BMDO) to obtain degradable, UCST copolymers
✅Synthesis by #RAFT
✅UCST (w/ MDO = no UCST)
✅Degradation in accelerated conditions
1/9
✅Synthesis by #RAFT
✅UCST (w/ MDO = no UCST)
✅Degradation in accelerated conditions
1/9
https://twitter.com/julnicolas/status/1529055420559593473

The UCST can be finely tuned by simply varying the amount of CKA in the copolymer. With BMDO, Tcp = 22-55°C, thus covering r.t. and the body temperature, and opening the door to biomedical applications 💊 #hyperthermia 2/9 

🔴 We also found that these copolymers can be rapidly degraded under "physiological" conditions (PBS, pH 7.4, 37°C) and even in water, below or above their UCST.
How fast❓ Faster than #PLA and even #PLGA!
▶️Up to -70% decrease in Mn after 7 days! 3/9
How fast❓ Faster than #PLA and even #PLGA!
▶️Up to -70% decrease in Mn after 7 days! 3/9

Why do we think this is so cool? It’s because CKA-containing copolymers have always suffered from slow hydrolytic degradation under physiological conditions, e.g. -70% decrease in Mn for OEGMA/MPDL copolymers after 12… months!
4/9
pubs.acs.org/doi/10.1021/ac…
4/9
pubs.acs.org/doi/10.1021/ac…
Therefore, we believe that the AAm/CKA copolymerization system could open new exciting perspectives in the field of degradable vinyl polymers. 5/9
But there’s…
But there’s…

You can synthesize POEGMA-b-P(AAm-co-BMDO) amphiphilic diblock copolymers that exhibit both #UCST and #LCST transitions owing to the LCST of POEGMA, and the 3 different solubility states can be reversibly reached by oscillating the temperature (e.g., from 5 to 85°C). 6/9
📽
📽
Icing on the cake: such copolymers can be formulated into degradable, #PEGylated, UCST nanoparticles by an *all-water* #nanoprecipitation process. Warm water poured into cold water, no organic solvent. 7/9 👇 
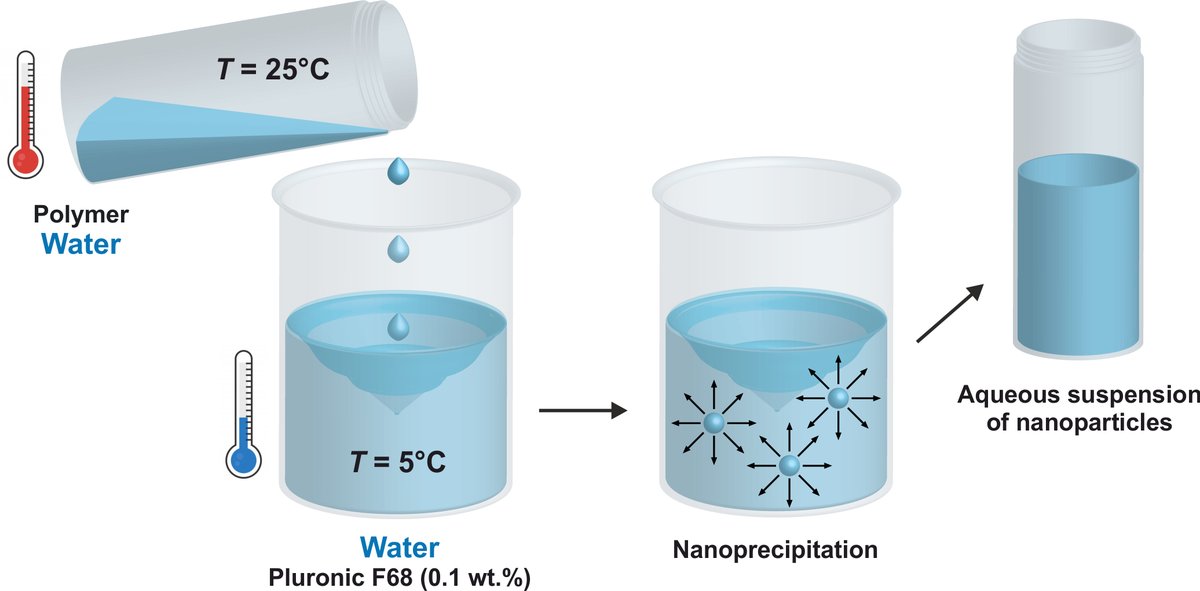
✅The nanoparticles exhibit both UCST and LCST transitions
✅Their colloidal characteristics are reversible upon oscillating the temperature 8/9
✅Their colloidal characteristics are reversible upon oscillating the temperature 8/9

Thanks for reading, hope you enjoyed the 🧵 !
And last but not least, the first author Amaury Bossion (poke @SardonL @DavidMecerreyes @POLYMAT_BERC) has recently found a permanent position at @Huntsman_Corp in Bruxelles! 🥳 9/9
And last but not least, the first author Amaury Bossion (poke @SardonL @DavidMecerreyes @POLYMAT_BERC) has recently found a permanent position at @Huntsman_Corp in Bruxelles! 🥳 9/9
Please unroll @threadreaderapp
• • •
Missing some Tweet in this thread? You can try to
force a refresh



