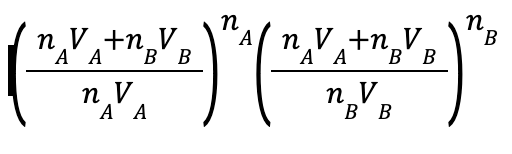#thermofact
Thermodynamics of phase transition
1/10 This thread focuses on a simple case, which illustrates the thermodynamic method.
Make a system of one species of molecules.
Neglect change in volume.
Thus, the system has a single independent variable: energy.
Thermodynamics of phase transition
1/10 This thread focuses on a simple case, which illustrates the thermodynamic method.
Make a system of one species of molecules.
Neglect change in volume.
Thus, the system has a single independent variable: energy.
2/10 Consider a system of H2O molecules and two phases, ice and water. Change in volume is small, and is neglected in this model.
Water is a phase, which can be in many states. The same is true for ice.
Water and ice can equilibrate in mixtures.
Water is a phase, which can be in many states. The same is true for ice.
Water and ice can equilibrate in mixtures.
3/10 Let u be the average energy per molecule, and s be the average entropy per molecule.
Draw a u-s plane.
On the u-s plane, each pure state corresponds to a point, and all pure states correspond to a set of points.
Draw a u-s plane.
On the u-s plane, each pure state corresponds to a point, and all pure states correspond to a set of points.

4/10 Two pure states, (uA, sA) and (uB, sB), form a mixture. The average energy and entropy (u,s) is a linear combination:
(u,s) = yA (uA, sA) + yB (uB, sB)
yA = fraction of molecules state A
yB = fraction of molecules state B
The mixture is a point on the segment.
(u,s) = yA (uA, sA) + yB (uB, sB)
yA = fraction of molecules state A
yB = fraction of molecules state B
The mixture is a point on the segment.

5/10 Similarly, a mixture of three pure states is a point in the triangle connecting the three pure states. 

6/10 One can similarly locate a mixture of any number of pure states.
Each mixture corresponds to a point on the u-s plane. Points of all mixtures are in a polygon, called the convex hull.en.wikipedia.org/wiki/Convex_hu…
Each mixture corresponds to a point on the u-s plane. Points of all mixtures are in a polygon, called the convex hull.en.wikipedia.org/wiki/Convex_hu…

7/10 The system is an isolated system if energy u is fixed, corresponding to a vertical line on the (u,s) plane.
The isolated system equilibrates by maximizes entropy s, corresponding to the interaction between the vertical line and the upper boundary of the convex hull.
The isolated system equilibrates by maximizes entropy s, corresponding to the interaction between the vertical line and the upper boundary of the convex hull.

8/10 As u changes, the system equilibrates in states corresponding to the upper boundary of the convex hull.
The system can equilibrium as either a pure state, or a mixture of two pure states.
The system cannot equilibrate as a mixture of three or more pure states.
The system can equilibrium as either a pure state, or a mixture of two pure states.
The system cannot equilibrate as a mixture of three or more pure states.

9/10 All pure states of a phase form a smooth curve.
On the u-s plane are a curve for ice (A), and a curve for water (B).
For the two curves of pure states, the convex hull consists of three pieces: part of curve A, part of curve B, and a tangent common to both curves.
On the u-s plane are a curve for ice (A), and a curve for water (B).
For the two curves of pure states, the convex hull consists of three pieces: part of curve A, part of curve B, and a tangent common to both curves.

10/10 Define temperature T by 1/T = ds(u)/du.
In a mixture of two pure states, the temperature of the two states are equal.
Approximate experimental data for ice and water are given in the T-s and T-u planes.

In a mixture of two pure states, the temperature of the two states are equal.
Approximate experimental data for ice and water are given in the T-s and T-u planes.


Writing this thread focussed me on essentials. I have updated my class notes under the heading "Thermodynamics of phase transition".
Rule of mixture implies convex hull.
An isolated system conserves energy and maximizes entropy.
docs.google.com/document/d/12j…
Rule of mixture implies convex hull.
An isolated system conserves energy and maximizes entropy.
docs.google.com/document/d/12j…
• • •
Missing some Tweet in this thread? You can try to
force a refresh