Very excited to share our latest research on immunological features of #LongCovid. Our 2+ year collaboration with @PutrinoLab with many other fantastic colleagues and patients - Mount Sinai Yale Long COVID (MY-LC) study by @sneakyvirus1 et al. 🧵(1/)
medrxiv.org/content/10.110…
medrxiv.org/content/10.110…
This work is led by the amazing @sneakyvirus1 with @wood_jamie_1 @_BlueJay3 @peowenlu @rahuldhodapkar @JeffGehlhausen @S_Tabachnikova from @aaronmring @david_van_dijk @PutrinoLab labs, with @hmkyale @SaadOmer3 @InciYildirim11 @RMedzhitov and @serimmune team & many others 👇🏽 (2/) 

There are multiple hypotheses behind long COVID pathogenesis including persistent virus/viral remnants, autoimmunity, dysbiosis, virome reactivation and tissue damage. Our data will dive deep into some of these. (3/)
science.org/doi/10.1126/sc…
science.org/doi/10.1126/sc…

This is a cross-sectional multi-dimensional immune phenotyping & patient surveys in people with or without LC, who got COVID during the 2020 first wave, on average more than a year from infection. Most were not-hospitalized, ♀ dominant, younger to middle age. (4/) 

Long COVID group reported significant increases in the intensity of symptoms and dramatically worsened quality of life. Survey outcomes put together into a single classification metric “Long COVID Propensity Score or LCPS” demonstrated significant diagnostic potential. (5/) 

Long COVID participants reported a number of symptoms, most commonly fatigue, brain fog, dysautonomia..etc. Hierarchical clustering of binary symptom data identified 3 clusters of patients with similar sets of self-reported symptoms. (6/) 

Next, flow cytometry analysis @peowenlu of peripheral blood mononuclear cells revealed several key differences in LC vs. CC or HC. First, LC had increase in non-conventional monocytes, activated B cells double-negative B cells, and decrease in conventional dendritic cells 1. (7/) 

Long COVID participants also had reduced central memory T cells and increased exhausted CD4 and CD8 T cells. The exhausted T cells suggest chronic antigens stimulating these T cells. What they are we do not know yet. (8/) 

Long COVID patients also had increases in CD4 T cells that secrete IL-2, IL-4 and IL-6, as well as some that secrete both IL-4 and IL-6. These T cells correlated with the levels of EBV reactive antibodies. Follow me down this thread further to find out more! (9/) 

We measured antibody levels against SARS-CoV-2 antigens in people who received 2 doses of mRNA vaccines. Curiously, long COVID patients produced higher levels of IgG against Spike. Without vaccines, LC had higher IgG against nucleocapsid. Data suggest persistent antigen? (10/) 



Not only did LC have higher levels of IgG against Spike, but also had IgG against distinct epitopes within the Spike protein, identified by @S_Tabachnikova with @serimmune Kathy Kamath. See the distinct peaks in purple vs. controls. (11/) 

Next we examined a large number of plasma factors and asked which factors are most different in long COVID vs. non-long COVID groups. By far the most significant differences were found in cortisol levels. Long COVID group had lower plasma cortisol levels than control groups.(12/) 

Cortisol levels in circulation were about half of the control groups. Despite this, we saw no elevation in ACTH levels, suggesting an impaired compensatory response by the hypothalamic-pituitary axis. (13/) 



This is so interesting, giving the report by @SuYapeng et al showing similar reduction in long haulers with respiratory viral symptoms at 2-3 months post COVID. This implies chronic hypocortisolism in long COVID. (14/) 

What about autoantibodies? @_BlueJay3 and @aaronmring used Rapid Extracellular Antigen Profiling (REAP) version 2.0 (with more antigens added) to examine autoantibodies. In contrast to what we found for acute severe COVID, long COVID group did not have elevated AABs. (15/) 

However, REAP did find notably elevated autoantibodies to sodium ion transporters in a subset of Long COVID patients who displayed reactivities agains 6-9 different proteins of this family. Those with tinnitus and nausea had elevated levels of these AABs. (16/) 

In contrast to autoantibodies, REAP detected elevation in IgG against herpesvirus antigens. In particular, antibody reactivity to glycoproteins and early antigens of Epstein-Barr virus, Varicella zoster virus were elevated in long COVID over other groups. (17/) 
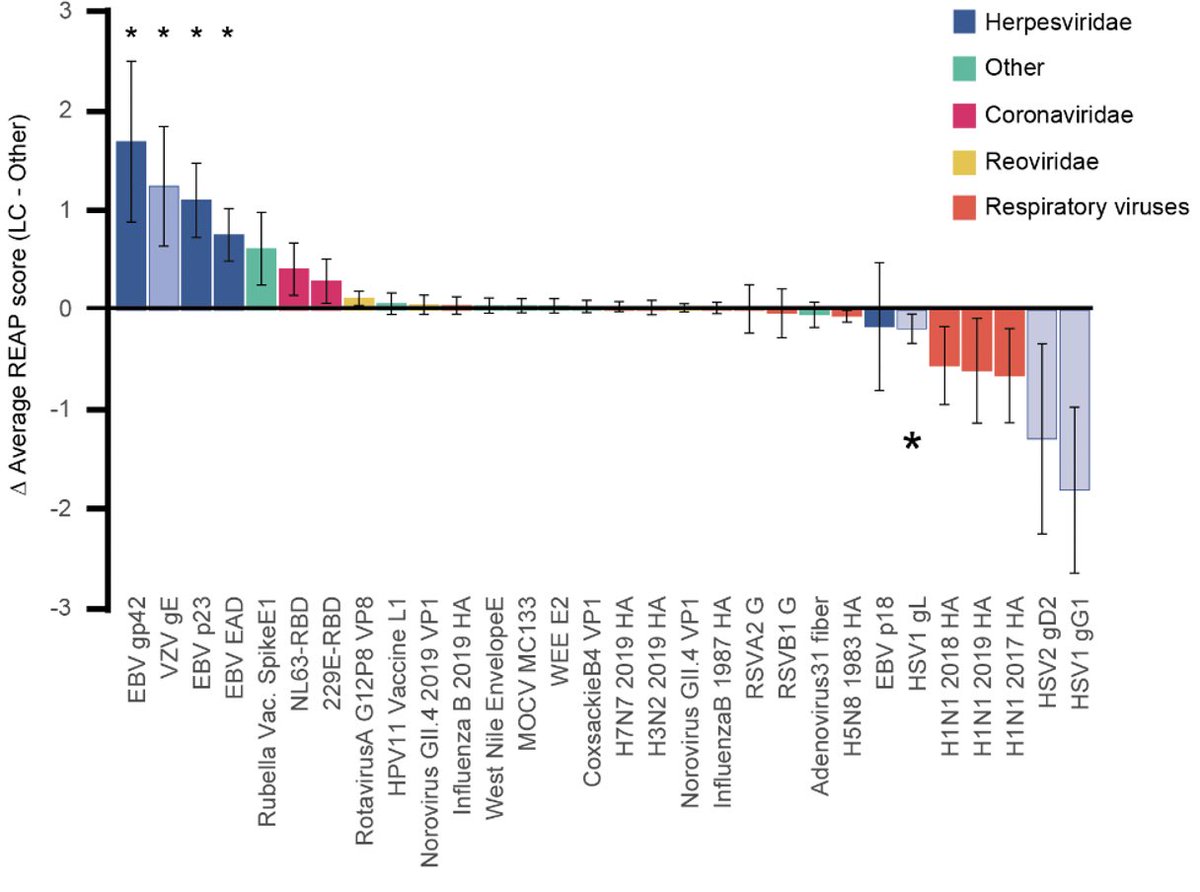
The increases in antibodies to EBV and VZV antigens were also detected using independent assays like ELISA and @serimmune epitope mapping. However, seroprevalence for EBV and VZV were similar in LC and CC. These data suggest recent reactivation of EBV and VZV in LC. (18/) 

This again is consistent with the report by @SuYapeng et al, showing that EBV viremia at the time of diagnosis is one of the four predictive factors for long COVID. (Note that our study did not examine viremia but infer EBV reactivation by serology)(19/)
sciencedirect.com/science/articl…
sciencedirect.com/science/articl…
Notably, these anti-EBV antibodies found in LC correlated with the levels of IL-4/IL-6 double-positive CD4 T cells I mentioned above. Significant correlations were also found between EBV p23 reactivity and terminally differentiated effector memory (TEMRA) CD4 T. (20/) 



Finally @rahuldhodapkar used machine learning and found that immune features alone can predict long COVID with efficient discriminative performance (AUC=0.96)! The most informative individual data blocks contributing to efficient separation of groups are flow and cytokine. (21/) 

Several features significantly distinguished Long COVID (double negative B cells, serum galectin-1, various EBV epitopes) while others were negatively associated (serum cortisol, PD-1+ CD4+ TCM, and HSV1 and HSV2 motifs). (22/) 

In fact, serum cortisol was the most significant individual predictor of Long COVID status in the model, and alone was a predictor wit an AUC of 0.96 (95% CI: 0.92-0.99). Notably, serum cortisol within the MY-LC study was highest in HC > CC > LC. (23/) 

We found many key circulating biological factors that alone can discriminate long COVID from others. Comparison of classification accuracies between patient reported outcomes and machine learning revealed substantial agreement (Cohen’s Kappa .865, 95% CI [0.83 - .90]). (24/)
While we are so excited about the findings of this study, we also wish to highlight key limitations of this study. They are summarized here but there may be more. Our study is exploratory in nature and requires validation. (25/) 

Here is the summary of our findings. There are many implications for diagnosis and therapy for #longCOVID. (26/) 

For example, can we target viral reservoir with antivirals and mAbs? Can we restore cortisol levels? Should therapy be targeting EBV? Would immunomodulatory therapy against inflammatory cytokines be useful? We still need to identify long COVID endotypes for treatment. (27/)
We hope that our study will be informative to others working in the field. We need validation across cohorts. We also hope that these data will help those who are still skeptical understand that long COVID is real, and it has biological basis. Thank you for reading. (End)
And I finally got to meet @PutrinoLab in person after a million zoom meetings and phone calls. How amazing to have a collaborator who is also a great friend!! 😊 

• • •
Missing some Tweet in this thread? You can try to
force a refresh